Chemistry of Iron
- Page ID
- 3720
Iron, which takes its English name from the old Anglo-Saxon and its symbol from the Latin, ferrum, was identified and used in prehistoric times. It is a very common element, fourth most abundant in the earth's crust. In addition, two of the ten most common compounds in the earth's crust are the two common oxides of iron, \(FeO\) and \(Fe_2O_3\).
Introduction
In its pure form, iron is a silvery-white metal, distinguished by its ability to take and retain a magnetic field, and also dissolve small amounts of carbon when molten (thus yielding steel). Commercial refining of iron is based on the heating of \(Fe_2O_3\) or \(Fe_3O_4\) (magnetite) with a mixture of other substances in the high temperature environment of the blast furnace. The oxides are reduced to pure iron. In addition to hardening iron by adding small amounts of carbon and also some other metals to the molten iron, iron castings or forgings can be heat-treated to take advantage of the various physical properties of the different solid phases of iron.
Pure iron reacts readily with oxygen and moisture in the environment and corrodes destructively. Even alloys such as steel need protection by painting or some other coating to prevent structural failure over time.
Iron as Catalyst
The Haber Process combines nitrogen and hydrogen into ammonia. The nitrogen comes from the air and the hydrogen is obtained mainly from natural gas (methane). Iron is used as a catalyst.
\[N_{2(g)} + 3H_{2(g)} \overset{Fe}{\rightleftharpoons} 2NH_{3(g)} \label{1} \]
The reaction between persulfate ions (peroxodisulfate ions), S2O82-, and iodide ions in solution can be catalyzed using either iron(II) or iron(III) ions. The overall equation for the reaction is:
\[ S_2O_8^{2-} + 2I^- \rightarrow 2SO_4^{2-}+ I_2 \label{2} \]
For the sake of argument, we'll take the catalyst to be iron(II) ions. The reaction happens in two stages.
\[ S_2O_8^{2-} + 2Fe^{2+} \rightarrow 2SO_4^{2-}+ 2Fe^{3+} \label{3} \]
\[ 2Fe^{3+} + 2I^- \rightarrow 2Fe^{2+}+ I_2 \label{4} \]
If you use iron(III) ions, the second of these reactions happens first. This is a good example of the use of transition metal compounds as catalysts because of their ability to change oxidation state.
Reactions of iron ions in solution
Free iron ions are compeled with water in aqueous solutions. The simplest of these complex ions are:
- the hexaaquairon(II) ion: \([Fe(H_2O)_6]^{2+}\).
- the hexaaquairon(III) ion: \([Fe(H_2O)_6]^{3+}\).
They are both acidic ions, but the iron(III) ion is more acidic.
Reactions of the iron ions with hydroxide ions
Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the iron ions. When enough hydrogen ions have been removed, you are left with a complex with no charge - a neutral complex. This is insoluble in water and a precipitate is formed.
In the iron(II) case:
\[[Fe(H_2O)_6]^{2+} + 2OH^- \rightarrow [Fe(H_2O)_4(OH)_2] + 2H_2O \label{5} \]
In the iron(III) case:
\[[Fe(H_2O)_6]^{3+} + 3OH^- \rightarrow [Fe(H_2O)_3(OH)_3] + 3H_2O \label{6} \]
In the test-tube, the color changes are:
- In the iron(II) case:
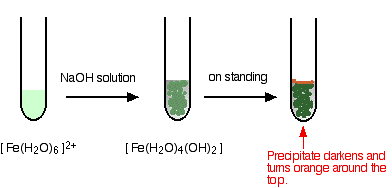
Iron is very easily oxidized under alkaline conditions. Oxygen in the air oxidizes the iron(II) hydroxide precipitate to iron(III) hydroxide especially around the top of the tube. The darkening of the precipitate comes from the same effect.
- In the iron(III) case:
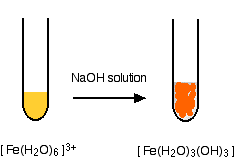
Reactions of Iron Ions with Ammonia
Ammonia can act as both a base and a ligand. In these cases, it simply acts as a base - removing hydrogen ions from the aqua complex.
In the iron(II) case:
\[ [Fe(H_2O)_6]^{2+} + 2NH_3 \rightarrow [Fe(H_2O)_4(OH)_2] + 2NH_4^+ \nonumber \]
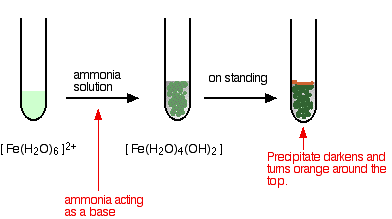
The appearance is just the same as in when you add sodium hydroxide solution. The precipitate again changes color as the iron(II) hydroxide complex is oxidized by the air to iron(III) hydroxide. In the iron(III) case:
\[ [Fe(H_2O)_6]^{3+} + 3NH_3 \rightarrow [Fe(H_2O)_3(OH)_3] + 3NH_4^+ \nonumber \]
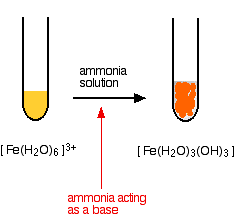
The reaction looks just the same as when you add sodium hydroxide solution.
Reactions of the iron ions with carbonate ions
There is an important difference here between the behavior of iron(II) and iron(III) ions.
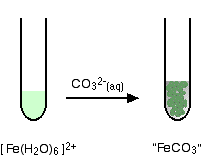
Iron(II) ions and carbonate ions
You simply get a precipitate of what you can think of as iron(II) carbonate.
\[ Fe^{2+} (aq) + CO_3^{2-} \rightarrow FeCO_3(s) \nonumber \]
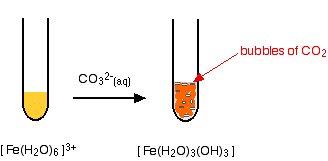
Iron(III) ions and carbonate ions
The hexaaquairon(III) ion is sufficiently acidic to react with the weakly basic carbonate ion. If sodium carbonate solution is added to a solution of hexaaquairon(III) ions, you get exactly the same precipitate as if you added sodium hydroxide solution or ammonia solution. This time, it is the carbonate ions which remove hydrogen ions from the hexaaqua ion and produce the neutral complex. Depending on the proportions of carbonate ions to hexaaqua ions, you will get either hydrogencarbonate ions formed or carbon dioxide gas from the reaction between the hydrogen ions and carbonate ions. The more usually quoted equation shows the formation of carbon dioxide.
\[2 [Fe(H_2O)_6^{3+} + 3CO_3^{2-} \rightarrow 2[Fe(H_2O)_3(OH)_3] + 3CO_2 + 3H_2O \nonumber \]
Apart from the carbon dioxide, there is nothing new in this reaction:
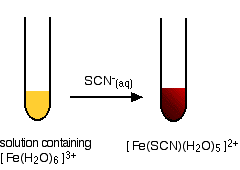
Testing for iron(III) ions with thiocyanate ions
This provides an extremely sensitive test for iron(III) ions in solution. If you add thiocyanate ions, SCN-, (e.g., from sodium or potassium or ammonium thiocyanate solution) to a solution containing iron(III) ions, you get an intense blood red solution containing the ion [Fe(SCN)(H2O)5]2+.
Finding the concentration of iron(II) ions in solution by Redox titration
You can find the concentration of iron(II) ions in solution by titrating with either potassium manganate(VII) solution or potassium dichromate(VI) solution. The reactions are done in the presence of dilute sulfuric acid. In either case, you would pipette a known volume of solution containing the iron(II) ions into a flask, and add a roughly equal volume of dilute sulfuric acid. What happens next depends on whether you are using potassium manganate(VII) solution or potassium dichromate(VI) solution.
Using potassium manganate(VII) solution
The potassium manganate(VII) solution is run in from a burette. At first, it turns colorless as it reacts. The end point is the first trace of permanent pink in the solution showing a tiny excess of manganate(VII) ions. The manganate(VII) ions oxidize iron(II) to iron(III) ions. The two half-equations for the reaction are:
\[ Fe^{2+} \rightarrow Fe^{3+} + e^- \label{10} \]
\[ MnO_4^- + 8H^+ + 5e^- \rightarrow Mn^{2+} + 4H_2O \label{11} \]
These combine to give the ionic equation for the reaction:
\[ 5Fe^{2+} + MnO_4^- + 8H^+\rightarrow Mn^{2+} + 4H_2O + 5Fe^{3+} \label{12} \]
The complete equation shows that 1 mole of manganate(VII) ions react with 5 moles of iron(II) ions. Having got that information, the titration calculations are just like any other ones.
Using potassium dichromate(VI) solution
Potassium dichromate(VI) solution turns green as it reacts with the iron(II) ions, and there is no way you could possibly detect the color change when you have one drop of excess orange solution in a strongly colored green solution. With potassium dichromate(VI) solution you have to use a separate indicator, known as a redox indicator. These change color in the presence of an oxidizing agent. There are several such indicators - such as diphenylamine sulfonate. This gives a violet-blue color in the presence of excess potassium dichromate(VI) solution.
The two half-equations are:
\[ Fe^{2+} \rightarrow Fe^{3+} + e^- \label{13} \]
\[ Cr_2O_7^{2-} + 14H^+ + 6e^- \rightarrow 2Cr^{3+} + 7H_2O \label{14} \]
Combining these gives:
\[ Cr_2O_7^{2-} + 14H^+ + 6Fe^{2+} \rightarrow 2Cr^{3+} + + 7H_2O + 6Fe^{3+} \label{15} \]
You can see that the reacting proportions are 1 mole of dichromate(VI) ions to 6 moles of iron(II) ions. Once you have established that, the titration calculation is again going to be just like any other one.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)
Stephen R. Marsden


