Physical Properties of Period 3 Elements
- Page ID
- 3662
This page describes and explains the trends in atomic and physical properties of the Period 3 elements from sodium to argon. It covers ionization energy, atomic radius, electronegativity, electrical conductivity, melting point and boiling point.
Electronic structures
Across Period 3 of the Periodic Table, the 3s and 3p orbitals fill with electrons. Below are the abbreviated electronic configurations for the eight Period 3 elements:
| Na | [Ne] 3s1 |
| Mg | [Ne] 3s2 |
| Al | [Ne] 3s2 3px1 |
| Si | [Ne] 3s2 3px1 3py1 |
| P | [Ne] 3s2 3px1 3py1 3pz1 |
| S | [Ne] 3s2 3px2 3py1 3pz1 |
| Cl | [Ne] 3s2 3px2 3py2 3pz1 |
| Ar | [Ne] 3s2 3px2 3py2 3pz2 |
In each case, [Ne] represents the complete electronic configuration of a neon atom.
First Ionization Energy
The first ionization energy is the energy required to remove the most loosely held electron from one mole of gaseous atoms to produce 1 mole of gaseous ions each with a charge of +1.
\[ X (g) \rightarrow X^+ (g) + e^- \nonumber \]
The molar first ionization energy is the energy required to carry out this change per mole of \(X\).
The pattern of first ionization energies across Period 3
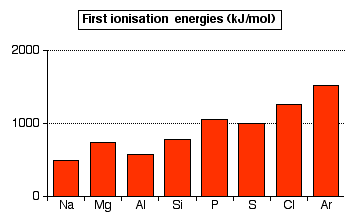
There is a general upward trend across the period, but this trend is broken by decreases between magnesium and aluminum, and between phosphorus and sulfur.
Explaining the pattern
First ionization energy is dependent on four factors:
- the charge on the nucleus;
- the distance of the outer electron from the nucleus;
- the amount of screening by inner electrons;
- whether the electron is alone in an orbital or one of a pair.
The upward trend: In the whole of period 3, the outer electrons are in 3-level orbitals. These electrons are at approximately the same distance from the nucleus, and are screened by corresponding electrons in orbitals with principal atomic numbers n=1 and n=2. The determining factor in the increase in energy is the increasing number of protons in the nucleus from sodium across to argon. This creates greater attraction between the nucleus and the electrons and thus increases the ionization energies. The increasing nuclear charge also pulls the outer electrons toward the nucleus, further increasing ionization energies across the period.
The decrease at aluminum: The value for aluminum might be expected to be greater than that of magnesium due to the extra proton. However, this effect is offset by the fact that the outer electron of aluminum occupies a 3p orbital rather than a 3s orbital. The 3p electron is slightly farther from the nucleus than the 3s electron, and partially screened by the 3s electrons as well as the inner electrons. Both of these factors offset the effect of the extra proton.
The decrease at sulfur: In this case something other than the transition from a 3s orbital to a 3p orbital must offset the effect of an extra proton. The screening (from the inner electrons and, to some extent, from the 3s electrons) is identical in phosphorus and sulfur , and the electron is removed from an identical orbital. The difference is that in the case of sulfur, the electron being removed is one of the 3px2 pair. The repulsion between the two electrons in the same orbital creates a higher-energy environment, making the electron easier to remove than predicted.
Atomic radius
The diagram below shows how atomic radius changes across Period 3.
The figures used to construct this diagram are based on:
- metallic radii for Na, Mg and Al;
- covalent radii for Si, P, S and Cl;
- the van der Waals radius for Ar (which forms no strong bonds).
It is appropriate to compare metallic and covalent radii because they are both being measured in tightly bonded circumstances. These radii cannot be compared with a van der Waals radius, however, making the diagram deceptive. The general trend towards smaller atoms across the period is not broken at argon. For convenience and clarity, argon is ignored in this discussion.
Explaining the Trend
A metallic or covalent radius is a measure of the distance from the nucleus to the bonding pair of electrons. From sodium to chlorine, the bonding electrons are all in the 3-level, screened by the electrons in the first and second levels. The increasing number of protons in the nucleus across the period attracts the bonding electrons more strongly. The amount of screening is constant across Period 3.
Electronegativity
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is most commonly used. Fluorine (the most electronegative element) is assigned a value of 4.0, and values decrease toward cesium and francium which are the least electronegative at 0.7.
The trend
The trend across Period 3 looks like this:

Argon is not included; because it does not form covalent bonds, its electronegativity cannot be assigned.
Explaining the Trend
The explanation is the same as that for the trend in atomic radii. Across the period, the valence electrons for each atom are in the 3-level. They are screened by the same inner electrons. The only difference is the number of protons in the nucleus. From sodium to chlorine, the number of protons steadily increases and so attracts the bonding pair more closely.
Physical Properties
This section discusses electrical conductivity and the melting and boiling points of the Period 3 elements. An understanding of the structure of each element is necessary for this discussion.
Structures of the elements
The structures of the elements vary across the period. The first three are metallic, silicon is network covalent, and the rest are simple molecules.
Three metallic structures
Sodium, magnesium and aluminum all have metallic structures. In sodium, only one electron per atom is involved in the metallic bond, the single 3s electron. In magnesium, both of its outer electrons are involved, and in aluminum all three are involved. One key difference to be aware of is the way the atoms are packed in the metal crystal. Sodium is 8-coordinated with each sodium atom interacting with only 8 other atoms. Magnesium and aluminum are each 12-coordinated, and therefore packed more efficiently, creating less empty space in the metal structures and stronger bonding in the metal.
A network covalent structure
Silicon has a network covalent structure like that of diamond. A representative section of this structure is shown:
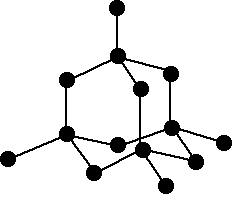
The structure is held together by strong covalent bonds in all three dimensions.
Four simple molecular structures
The structures of phosphorus and sulfur vary depending on the type of phosphorus or sulfur in question. In this case, white phosphorus and one of the crystalline forms of sulfur—rhombic or monoclinic—are considered. These structures are shown below:

Aside from argon, the atoms in each of these molecules are held together by covalent bonds. In the liquid or solid state, the molecules are held in close proximity by van der Waals dispersion forces .
Electrical conductivity
- Sodium, magnesium and aluminum are good conductors of electricity. Conductivity increases from sodium to magnesium to aluminum.
- Silicon is a semiconductor.
- Phosphorus, sulfur, chlorine, and argon are nonconductive.
The three metals conduct electricity because the delocalized electrons (as in the "sea of electrons" model) are free to move throughout the solid or the liquid metal. Semiconductor chemistry for substances such as silicon is beyond the scope of most introductory level chemistry courses. The other elements do not conduct electricity because they are simple molecular substances. without free, delocalized electrons..
Melting and boiling points
The chart shows how the melting and boiling points of the elements change as you go across the period. The figures are plotted in kelvin rather than °C to avoid showing negative temperatures.
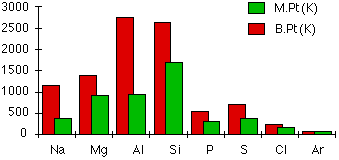
The metallic structures
Melting and boiling points increase across the three metals because of the increasing strength of their metallic bonds. The number of electrons which each atom can contribute to the delocalized "sea of electrons" increases. The atoms also get smaller and have more protons as you go from sodium to magnesium to aluminum. The attractions and therefore the melting and boiling points increase because:
- The nuclei of the atoms are more positively charged.
- The "sea" is more negatively charged.
- The "sea" is progressively nearer to the nuclei and thus is more strongly attracted.
Silicon
Silicon has high melting and boiling points due to its network covalent structure. Melting or boiling silicon requires the breaking of strong covalent bonds. Because of the two different types of bonding in silicon and aluminum, it makes little sense to directly compare the two melting and boiling points.
The four molecular elements
Phosphorus, sulfur, chlorine and argon are simple molecular substances with only van der Waals attractions between the molecules. Their melting or boiling points are lower than those of the first four members of the period which have complex structures. The magnitudes of the melting and boiling points are governed entirely by the sizes of the molecules, which are shown again for reference:

- Phosphorus: Elemental phosphorus adopts the tetrahedral P4 arrangement. Melting phosphorus breaks no covalent bonds; instead, it disrupts the much weaker van der Waals forces between the molecules.
- Sulfur: Elemental sulfur forms S8 rings of atoms. The molecules are bigger than phosphorus molecules, and thus the van der Waals attractions are stronger, leading to a higher melting and boiling point.
- Chlorine: Chlorine, Cl2, is a much smaller molecule with comparatively weak van der Waals attractions, and thus chlorine will have a lower melting and boiling point than sulfur or phosphorus.
- Argon: Elemental argon is monatomic . The scope for van der Waals attractions between argon atoms is very limited and so the melting and boiling points of argon are lower again.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)


