Halogens as Oxidizing Agents
- Page ID
- 3697
This page examines the trend in oxidizing ability of the Group 17 elements (the halogens): fluorine, chlorine, bromine and iodine. It considers the ability of one halogen to oxidize the ions of another, and how this changes down the group.
Basic facts
Consider a situation in which one halogen (chlorine, for example) is reacted with the ions of another (iodide, perhaps) from a salt solution. In the chlorine and iodide ion case, the reaction is as follows:
\[\ce{Cl_2 + 2I^- \rightarrow 2Cl^- + I_2} \nonumber \]
- The iodide ions lose electrons to form iodine molecules. In other words, they are oxidized.
- The chlorine molecules gain electrons to form chloride ions— they are reduced.
This is therefore a redox reaction in which chlorine acts as an oxidizing agent.
Fluorine
Fluorine must be excluded from this discussion because its oxidizing abilities are too strong. Fluorine oxidizes water to oxygen, as in the equation below, and so it is impossible to carry out reactions with it in aqueous solution.
\[\ce{2F_2 + 2H_2O \rightarrow 4HF + O_2} \nonumber \]
Chlorine, Bromine and Iodine
In each case, a halogen higher in the group can oxidize the ions of one lower down. For example, chlorine can oxidize bromide ions to bromine:
\[\ce{Cl_2 + 2Br^- \rightarrow 2Cl^- + Br_2} \nonumber \]
The bromine forms an orange solution. As shown below, chlorine can also oxidize iodide ions to iodine:
\[\ce{Cl_2 +2I^- \rightarrow 2Cl^- + I_2} \nonumber \]
The iodine appears either as a red solution if little chlorine is used, or as a dark gray precipitate if the chlorine is in excess.
Bromine can only oxidize iodide ions, and is not a strong enough oxidizing agent to convert chloride ions into chlorine. A red solution of iodine is formed (see the note above) until the bromine is in excess. Then a dark gray precipitate is formed.
\[\ce{Br_2 + 2I^- \rightarrow 2Br^- + I_2} \nonumber \]
Iodine won't oxidize any of the other halide ions, except possibly the extremely radioactive and rare astatide ions.
To summarize
- Oxidation is the loss of electrons. Each of the elements (for example, chlorine) could potentially take electrons from something else and are subsequently ionized (e.g., Cl-). This means that they are all potential oxidizing agents.
- Fluorine is such a powerful oxidizing agent that solution reactions are unfeasible.
- Chlorine has the ability to take electrons from both bromide ions and iodide ions. Bromine and iodine cannot reclaim those electrons from the chloride ions formed.
- This indicates that chlorine is a more powerful oxidizing agent than either bromine or iodine.
- Similarly, bromine is a more powerful oxidizing agent than iodine. Bromine can remove electrons from iodide ions, producing iodine; iodine cannot reclaim those electrons from the resulting bromide ions.
In short, oxidizing ability decreases down the group.
Explaining the trend
Whenever one of the halogens is involved in oxidizing a species in solution, the halogen end is reduced to a halide ion associated with water molecules. The following figure illustrates this process:
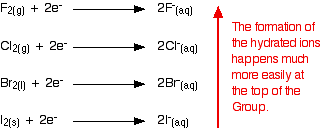
Down the group, the ease with which these hydrated ions are formed decreases; the halogens become less effective as oxidizing agents, taking electrons from something else less readily. The reason that the hydrated ions form less readily down the group is due to several complicated factors. Unfortunately, this explanation is often over-simplified, giving a faulty and misleading explanation. The wrong explanation is dealt with here before a proper explanation is given.
The Incorrect Explanation
The following explanation is normally given for the trend in oxidizing ability of chlorine, bromine and iodine. The ease of ionization depends on how strongly the new electrons are attracted. As the atoms get larger, the new electrons are further from the nucleus and increasingly shielded by the inner electrons (offsetting the effect of the greater nuclear charge). The larger atoms are therefore less effective at attracting new electrons and forming ions. This is equivalent to saying electron affinity decreases down the group. Electron affinity is described in detail on another page.
The problem with this argument is that it does not include fluorine. Fluorine's tendency to form a hydrated ion is much higher than that of chlorine. However, fluorine's electron affinity is less than that of chlorine. This contradicts the above argument. This problem stems from examining a single part of a very complicated process. The argument about atoms accepting electrons applies only to isolated atoms in the gas state picking up electrons to form isolated ions, also in the gas state. The argument must be generalized.
In reality:
- The halogen starts as a diatomic molecule, X2. This may be a gas, liquid or solid at room temperature, depending on the halogen.
- The diatomic molecule must split into individual atoms (atomization)
- Each atom gains an electron (electron affinity; this is the element of the process of interest in the faulty explanation.)
- The isolated ions are surrounded by water molecules; hydrated ions are formed (hydration).
The Correct Explanation
The table below shows the energy involved in each of these changes for atomization energy, electron affinity, and hydration enthalpy (hydration energy):
| atomization energy (kJ mol-1) |
electron affinity (kJ mol-1) |
hydration enthalpy (kJ mol-1) |
overall (kJ mol-1) |
|
|---|---|---|---|---|
| F | +79 | -328 | -506 | -755 |
| Cl | +121 | -349 | -364 | -592 |
| Br | +112 | -324 | -335 | -547 |
| I | +107 | -295 | -293 | -481 |
Consider first the fifth column, which shows the overall heat evolved, the sum of the energies in the previous three columns.
The amount of heat evolved decreases quite dramatically from the top to the bottom of the group, with the biggest decrease between fluorine and chlorine. Fluorine generates a large amount of heat when it forms its hydrated ion, chlorine a lesser amount, and so on down the group.
The first electron affinity is defined as the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions, as in the following equation: In symbol terms:
\[ X(g) + e^- \rightarrow X^-(g) \nonumber \]
The fifth column measures the energy released when 1 mole of gaseous ions dissolves in water to produce hydrated ions, as in the following equation, which is not equivalent to that above:
\[ X^-(g) \rightarrow X^- (aq) \nonumber \]
Why is fluorine a stronger oxidizing agent than chlorine?
There are two main factors. First, the atomization energy of fluorine is abnormally low. This reflects the low bond enthalpy of fluorine.
The main reason, however, is the very high hydration enthalpy of the fluoride ion. That is because fluoride is very small. There is a very strong attraction between fluoride ions and water molecules. The stronger the attraction, the more heat is evolved when the hydrated ions are formed.
Why does oxidizing ability decrease from chlorine to bromine to iodine?
The decrease in atomization energy between these three elements is relatively small, and would tend to make the overall change more negative down the group. It is helpful to look at the changes in electron affinity and hydration enthalpy down the group. Using the figures from the previous table:
| change in electron affinity (kJ mol-1) |
change in hydration enthalpy (kJ mol-1) |
|
|---|---|---|
| Cl to Br | +25 | +29 |
| Br to I | +29 | +42 |
Both of these effects contribute, but the more important factor—the one that changes the most—is the change in the hydration enthalpy. Down the group, the ions become less attractive to water molecules as they get larger. Although the ease with which an atom attracts an electron matters, it is not as important as the hydration enthalpy of the negative ion formed.
The faulty explanation is incorrect even if restricted to chlorine, bromine and iodine:
- This is the energy needed to produce 1 mole of isolated gaseous atoms starting from an element in its standard state (gas for chlorine, and liquid for bromine, for example, both of the form X2).
- For a gas like chlorine, this is simply half of the bond enthalpy (because breaking a Cl-Cl bond produces 2 chlorine atoms, not 1). For a liquid like bromine or a solid like iodine, it also includes the energy that is needed to convert them into gases.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)


