8.5.1: Preparation and General Properties of the Alkaline Earth Elements
- Page ID
- 199690
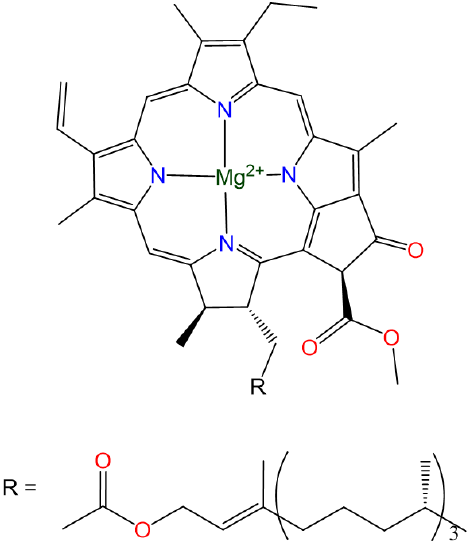
Like the alkali metals, the alkaline earth metals are good reductants that are only found on Earth in their +2 oxidation states. Magnesium and calcium are particularly common. Magnesium-containing chlorophylls are the photosynthetic pigments of green plants and photosynthetic algae (Scheme \(\sf{\PageIndex{1}}\)).
Scheme \(\sf{\PageIndex{1}}\). Structure of chlorophyll a, one of the principle green pigments of plants.
Seawater is about 0.05 M in Mg2+ and 0.01 M in Ca2+, and beryllium and calcium are components of many important minerals. Examples of alkaline earth metal-bearing minerals that were known and used since antiquity include beryl, Be3Al2Si6O18; gypsum, CaSO4·2H2O; limestone, CaCO3; and lime, CaO, the latter of which were more recently replaced in construction by calcium silicate-based sand lime bricks and Portland cement containing concrete.
Despite calcium and magnesium's widespread environmental distribution and importance, the metals were only isolated in the late 18th and early 19th century after the development of electrolysis. Humphrey Davy's isolation of Mg and Ca is illustrative.
\[\sf{2~MO~~\overset{electrolysis}{\longrightarrow}~~2~M(s)~~+~~O_2~~~~~~Davy, (M~=~Mg,~Ca,~1808)} \nonumber \]
Today Ca, Mg, and the other alkaline earth metals are produced either by electrolysis of their chlorides or chemical reduction by carbon or aluminum.
\[\sf{2~MCl_2~~\overset{electrolysis}{\longrightarrow}~~2~M(s)~~+~~Cl_2~~~~~~(M~=~Be,~Mg,~Ca,~Sr)} \nonumber \]
\[\sf{MgO~~+~~C~~\overset{electrolysis}{\longrightarrow}~~Mg(s)~~+~~CO} \nonumber \]
\[\sf{3~MO~~+~~2~Al~~\overset{electrolysis}{\longrightarrow}~~M(s)~~+~~Al_2O_3~~~~~~(M~=~Ca,~Sr,~Ra)} \nonumber \]
\[\sf{4~BaO~~+~~2~Al~~\overset{electrolysis}{\longrightarrow}~~Ba(s)~~+~~BaAl_2O_4} \nonumber \]
Once formed, the metals are reactive towards atmospheric oxygen. Nevertheless, Be and Mg may be stored and used in air since their initial oxidation gives a thin passivating layer of the oxide that seals off the bulk metal from further oxidation. The other alkaline earth metals need to be stored under an inert atomosphere to prevent their degradation to the oxide.
Physical Properties
Like the alkali metals, the alkaline earth metals are all grey or silvery solids that crystallize in a cubic lattice, HCP for Be and Mg, FCC/CCP for Ca and Sr, and BCC for Ba and Ra. All possess the typical metallic properties of high heat and thermal conductivity. Aside from the lightest member of the group, they are comparatively low melting and soft on account of their large atomic radii, low effective nuclear charges, and just two valence electrons that contribute to metallic bonding. Thus they melt and boil at higher temperatures than the alkali metals but lower temperatures than most transition metals, as illustrated by the melting and boiling points listed in Table \(\sf{\PageIndex{1}}\). As with the alkali metals, the melting and boiling points decrease down the group as the atomic size increases.
Table \(\sf{\PageIndex{1}}\). Melting and boiling points of the alkaline earth metals and selected reference substances.3
| Substance | Melting Point (\(^{\circ}\) C) | Boiling Point (\(^{\circ}\) C) |
| Alkaline Earth Metal | ||
| Beryllium, Be | 1278 | 2468 |
| Magnesium, Mg | 649 | 1090 |
| Calcium, Ca | 839 | 1484 |
| Strontium, Sr | 769 | 1384 |
| Barium, Ba | 727 | 1845 |
| Radium, Ra | 700 | 1140 |
| Non-alkaline Earth Metal | ||
| Lithium, Li | 181 | 1347 |
| Sodium, Na | 98 | 883 |
| Cesium, Cs | 28 | 678 |
| Titanium, Ti | 1660 | 3287 |
| Iron, Fe | 1538 | 2861 |
| Copper, Cu | 1083 | 2567 |
| Water | 0 | 100 |
| Benzene | 6 | 80 |
The atomic properties of alkaline earth metals reflect the
- relatively high energy and large size of their ns orbitals
- higher effective nuclear charge experienced by electrons in their ns valence orbitals when compared to those of the corresponding alkali metals
As may be seen from the atomic radii and ionization energies given in Table \(\sf{\PageIndex{2}}\), overall the radii and ionization energies of the alkaline earth metals follow the expected periodic trends. The radii increase down a group, and across a row they follow the trend
\[\sf{alkali~metal~>~alkaline~earth~metal~>~transition~metals} \nonumber \]
Because of their smaller size and larger atomic mass, alkaline earth metals are significantly more dense than the corresponding alkali metals (Table \(\sf{\PageIndex{2}}\)).
Correspondingly, the ionization energies of the alkaline earth metals decrease down a group while across a row their ionization energies follow the trend
\[\sf{alkali~metal~<~alkaline~earth~metal~<~transition~metals} \nonumber \]
In consequence the alkaline earth metals are good reductants and prefer to form the +2 ion, although they are not as reactive as the alkali metals. Moreover, Be possesses an anomalously small radius, high ionization energy, and large electronegativity when compared with heavier alkaline earth metals. Consequently, its chemical behavior is similar to that of boron and aluminum, as will be discussed in the next section.
Table \(\sf{\PageIndex{2}}\). Selected atomic properties of the alkali metals and selected reference compounds.3,4
| Substance |
Atomic Radius (Angstroms) |
First Ionization Energy (kJ/mol) |
Pauling Electronegativity |
Density of the Solid (g/mL) |
| Alkaline Earth Metal | ||||
| Beryllium, Be | 1.05 | 899 | 1.57 | 1.85 |
| Magnesium, Mg | 1.50 | 738 | 1.31 | 1.74 |
| Calcium, Ca | 1.80 | 590 | 1.00 | 1.55 |
| Strontium, Sr | 2.00 | 550 | 0.95 | 2.54 |
| Barium, Ba | 2.15 | 503 | 0.89 | 3.59 |
| Radium, Ra | 2.15 | 509 | 0.89 | 5.0 |
| Non-alkaline Earth Metal | ||||
| Lithium, Li (same row as Be) | 1.45 | 513 | 0.98 | 0.53 |
| Sodium, Na (same row as Mg) | 1.80 | 496 | 0.93 | 0.97 |
| Potassium, K (same row as Ca) | 2.20 | 419 | 0.82 | 0.86 |
| Rubidium, Rb (same row as Sr) | 2.35 | 403 | 0.82 | 1.53 |
| Cesium, Cs (same row as Ba) | 2.60 | 376 | 0.79 | 1.87 |
| Francium. Fr (same row as Ra) | not determined | 400 | 0.7 | not determined |
| Titanium, Ti | 1.40 | 658 | 1.54 | 4.54 |
| Iron, Fe | 1.40 | 759 | 1.83 | 7.87 |
| Copper, Cu | 1.35 | 745 | 1.90 | 8.96 |
| Boron, B | 0.85 | 801 | 2.04 | 2.34 |
| Aluminum, Al | 1.25 | 577 | 1.61 | 2.70 |
| Nitrogen, N | 0.65 | 1402 | 3.04 | 1.03 (at 21 K) |
As will be explained in the next section, one of the factors that contributes to the reactivity of the alkali metals is their small ionization energies.
References and Notes
1. All physical and atomic property data for elements except atomic radii and the boiling and melting points of Ba, Be, and Fe are calculated from data in Emsley, J. The elements 2nd ed. Oxford University Press, 1991. Since the phase transition points for Be, Ba and Fe were either listed as correspoding to high pressure conditions or disagreed with values reported elsewhere they were taken from the data listed at https://www.rsc.org/periodic-table.
2. Atomic radii are the empirical radii determined by John C. Slater as reported in Slater, J. C. J. Chem. Phys. 1964, 41, 3199-3204. Values of these radii may be conveniently accessed at http://www.knowledgedoor.com/2/elements_handbook/slater_atomic-ionic_radius.html
Contributors and Attributions
Stephen Contakes, Westmont College

