8.5.2: Alkaline Earth Metals' Chemical Properties
- Page ID
- 199692
Alkaline earth metals are good reducing agents that tend to form the +2 oxidation state.
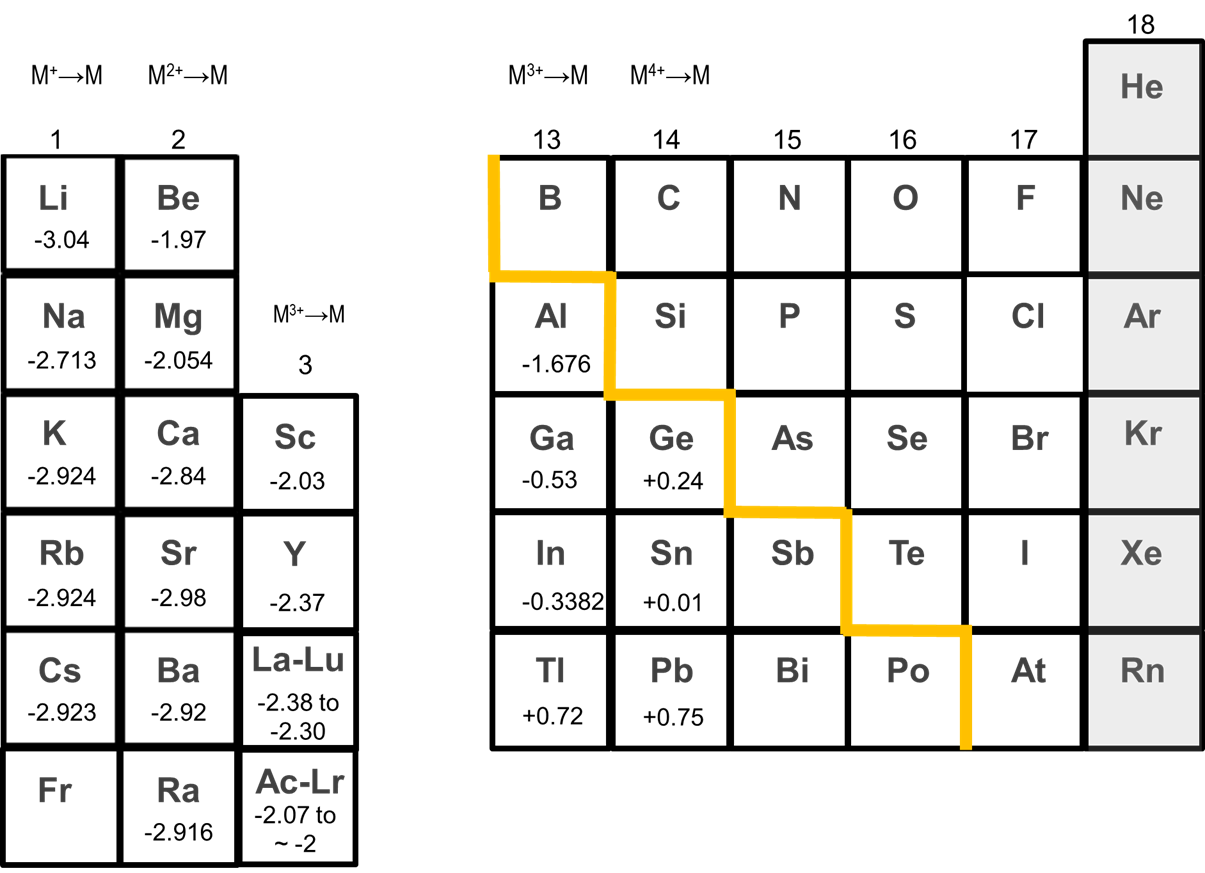
The alkaline earth metals tend to form +2 cations. As can be seen from Figure \(\sf{\PageIndex{2}}\), alkaline earth metals possess large negative M2+/0 standard reduction potentials which strongly favor the +2 cation. The potentials for reduction of Ca2+, Sr2+, and Ba2+ to the metal of ~ -3 V are even similar to those of the alkali metals.
Like the alkali metals, Ca, Sr, and Ba dissolve in liquid ammonia to give solutions containing solvated electrons, although these have not been as heavily studied as those of the alkali metals.
Unlike the alkali metals, all the alkaline earth metals react with oxygen to give oxides of formula MO, although the peroxides of the heavier alkaline earths can be formed by solution phase precipitation of the metal cation with a source of peroxide anion (O22-).
\[\sf{2~M(s)~~+~~O_2~\longrightarrow~~2~MO(s)~~~~~~~~~~~~~~~~~~~~~~~~~(M~=~Be,~Mg,~Ca,~Sr,~Ba,~and~presumably~Ra)} \nonumber \]
\[\sf{M(OH)_2(aq)~~+~~H_2O_2(aq)~\longrightarrow~~MO_2(s)~~+~~2~H_2O~~~~~~~~~~~~~~~~~~~~~~~~(M~=~Mg,~Ca,~Sr,~Ba,~and~presumably~Ra)} \nonumber \]
Calcium, strontium, barium (and presumably radium) react with water to liberate dihydrogen gas and form hydroxides.
\[\sf{M(s)~~+~~2~H_2O(l)~\rightarrow~M^{2+}~~+~~2~OH^-~~+~~H_2(g)~~~~~~~~~~~~~~~~~~~~~~~~~~(M~=~Ca,~Sr,~Ba,~and~presumably~Ra)} \nonumber \]
although unlike the alkali metals the reduction is slow and usually liberates hydrogen without fire or explosion.
Beryllium and magnesium do not react with water at room temperature, although they do dissolve in acid and react with steam at high temperatures and pressures to give the oxide (which can be thought of as a dehydrated hydroxide).
\[\sf{M(s)~~+~~2~H^+~~\rightarrow~M^{2+}~~+~~H_2(g)~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~(M~=~Be~and~Mg)} \nonumber \]
\[\sf{M(s)~~+~~H_2O(g)~~\overset{high~T,~P}{\longrightarrow}~MO(s)~~+~~H_2(g)~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~(M~=~Be~and~Mg)} \nonumber \]
Presumably Ca, Sr, Ba, and Ra would react this way as well, although due to their higher reactivity the reaction is likely to be violent.
Like other metal oxides containing low oxidation state metals, the alkaline earth oxides are basic. The oxides of the heavier alkaline earth metals react with water to give the hydroxides.
\[\sf{MO~~+~~H_2O~\longrightarrow~~M(OH)_2~~~~~~~~~~~~~~~~~~~~~~~~~~~~~(M~=~Be,~Mg,~Ca,~Sr,~Ba,~and~presumably~Ra)} \nonumber \]
The hydrolysis of BeO and MgO usually requires high temperatures and pressures. Consequently their hydroxides are more commonly prepared by addition of base to a soluble salt.
\[\sf{M^{2+}(aq)~~+~~2~OH^-~~\rightarrow~M(OH)_2~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~(M~=~Be,~Mg, Ca,~Sr,~Ba,~and~presumably~Ra)} \nonumber \]
The alkaline earth metals react directly with halogens to give the dihalides, although given the exothermicity of reactions involving the powerfully reducing alkaline earth metals with oxidizing halogens in the laboratory, it is usually safer to react the hydroxides or oxides with the appropriate hydrohalic acid.
\[\sf{M(s)~~+~~X_2^-~~\rightarrow~MX_2~~~~~~~~~~~~~~~~~~~~~~~~~~~~~(M~=~Be,~Mg, Ca,~Sr,~Ba,~and~presumably~Ra; X~=~F,~Cl,~Br,~I)} \nonumber \]
\[\sf{M(OH)_2~~+~~2~HX~~\rightarrow~MX_2~~+~~2~H_2O~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~(M~=~Mg, Ca,~Sr,~Ba,~and~presumably~Ra; X~=~F,~Cl,~Br,~I)} \nonumber \]
\[\sf{MO~~+~~2~HX~~\rightarrow~MX_2~~+~~H_2O~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~(M~=~Mg, Ca,~Sr,~Ba,~and~presumably~Ra; X~=~F,~Cl,~Br,~I)} \nonumber \]
The heavier alkaline earth metals (Mg through Ba) also reduce hydrogen to give hydrides.
\[\sf{M(s)~~+~~H_2(g)~~\rightarrow~MH_2~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~(M~=~Mg, Ca,~Sr,~Ba,~and~presumably~Ra)} \nonumber \]
As discussed in section 8.2.1. Hydrogen's Chemical Properties, these alkaline earth hydrides are ionic salts of hydride ion. Thus they react with water and other electrophiles.
\[\sf{MH_2(s)~~+~~H_2O(l)~~\rightarrow~M(OH)_2~~+~~H_2(g)~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~(M~=~Mg, Ca,~Sr,~Ba,~and~presumably~Ra)} \nonumber \]
The consumption of water in this reaction forms the basis for the use of calcium hydride as a drying agent for organic solvents.
Unlike the heavier alkaline earth metals, beryllium does not react directly with hydrogen, and the resulting hydride, though still nucleophilic, acts as a polar covalent hydride and hydrolyzes relatively slowly.
Divalent Alkaline Earth cations polarize anions
The classic example of alkaline earth cations' ability to polarize anions involves the decomposition of the metal carbonates. Alkali metal carbonates and nitrates thermally decompose to release CO2 and a mixture of NO2 and O2, respectively.
\[\sf{MCO_3(s)~~\overset{\Delta}{\longrightarrow}~MO(s)~~+~~CO_2(g)} \nonumber \]
\[\sf{M(NO_3)_2(s)~~\overset{\Delta}{\longrightarrow}~MO(s)~~+~~2~NO_2(g)~~+~~O_2(g)} \nonumber \]
Complex factors govern decomposition of the nitrates but, as shown in Table \(\sf{\PageIndex{1}}\), the decomposition of the alkaline earth carbonates shows that on going down the group, carbonate decomposition requires increasingly higher temperatures.
Table \(\sf{\PageIndex{1}}\). Decomposition temperatures of alkaline earth carbonates
| Carbonate |
Midpoint of decomposition range2 (K) |
| BeCO3 | not reported; unstable at room temperature (298 K) |
| MgCO3 | 903 |
| CaCO3 | 953 |
| SrCO3 | 1178 |
| BaCO3 | 1316 |
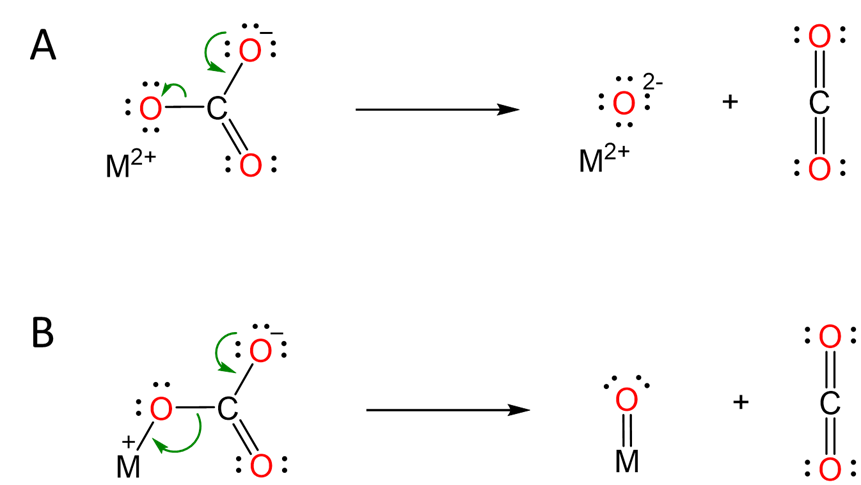
The typical explanation for this trend involves the mechanism of carbonate decomposition depicted in Scheme \(\sf{\PageIndex{II}}\). As the alkaline earth metal cation becomes larger on going from Be to Ba, its ability to polarize the carbonate anion is lessened, making it more difficult to form the oxide.
Scheme \(\sf{\PageIndex{I}}\). Models explaining how alkaline earth metal cations facilitate carbonate decomposition. (A) Ionic model in which the negative charge buildup is stabilized by interaction between the dication and one of the carbonate oxygens. (B) Semi-covalent representation of the same interaction, now depicted as a M=O bond (which should not be taken to imply that such a bond actually exists).
Alkaline earth metal sulfates undergo decomposition reactions similar to those of the carbonates and nitrates.
- Write a decomposition reaction that involves the liberation of a single molecular gas from the sulfate to give an oxide.
- Rank the alkaline earth metal sulfates in order of increasing decomposition temperature.
- Answer
-
1. The sulfates decompose by liberating SO3 according to the reaction
\[\sf{MSO_4(s)~~\overset{\Delta}{\longrightarrow}~MO(s)~~+~~SO_3(g)} \nonumber \]
Note that another possible decomposition mode is
\[\sf{MSO_4(s)~~\overset{\Delta}{\longrightarrow}~MO(s)~~+~~ {\textstyle \frac{1}{2}}~O_2(g)~~+~~SO_2(g)} \nonumber \]
However, that reaction is not the one asked for by the prompt since it involves the liberation of two different molecular gases (O2 and SO2).
2. The expected decomposition order of the alkaline earth metal sulfates along with known decomposition temperatures is
\[\sf{\underset{580~^{\circ}C}{BeSO_4}~<~\underset{895~^{\circ}C}{MgSO_4}~<~\underset{1149~^{\circ}C}{CaSO_4}~<~\underset{1374~^{\circ}C}{SrSO_4}~<~BaSO_4~<~RaSO_4} \nonumber \]
Beryllium, and to a lesser extent magnesium, form polar highly covalent compounds.
The chemistry of magnesium and beryllium demonstrates the danger of drawing an overly rigorous distinction between elements as metals, nonmetals, and metalloids. This is because both Be and Mg can form compounds with considerable covalent character and, as might be expected from their relative paucity of electrons, they share much in common with the electron deficient row 13 metalloids B and Al.
In terms of Mg, the influence of covalency is evident from the structures of the Grignard reagents that Mg forms on reaction between the metal and alkyl halides.
\[\sf{Mg(s)~~+~~R-X~~\overset{THF, catalytic~I_2}{\longrightarrow}~R-Mg-X} \nonumber \]
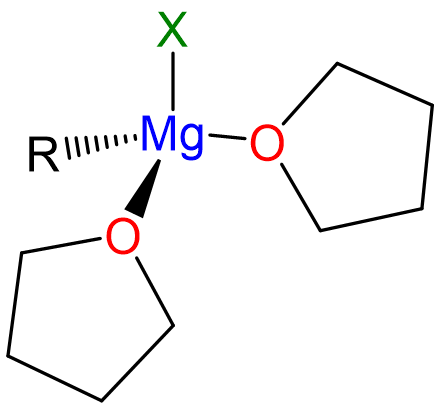
The reaction is dependent on the presence of Lewis base donor ethers like Et2O or THF, which coordinate the Mg2+, completing its coordination sphere and giving tetrahedral complexes like that depicted in Scheme \(\sf{\PageIndex{II}}\).
Scheme \(\sf{\PageIndex{II}}\). Structure of monomeric "RMgX" Grignard reagent in THF solution.
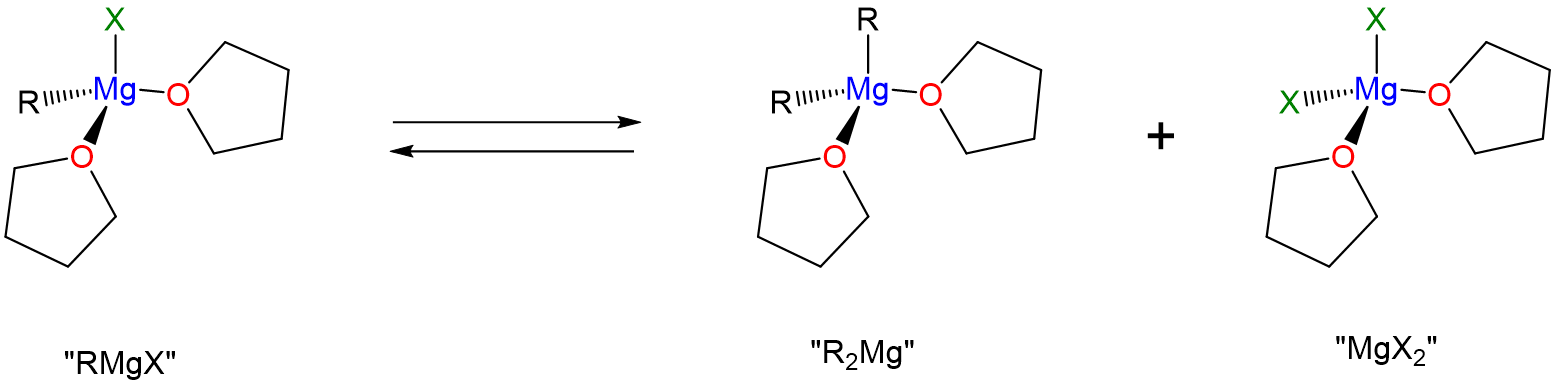
Like molecular compounds, Grignard reagents undergo ligand substitution reactions in solution according to the Schlenk equilibrium.
 \[ \nonumber \]
\[ \nonumber \]
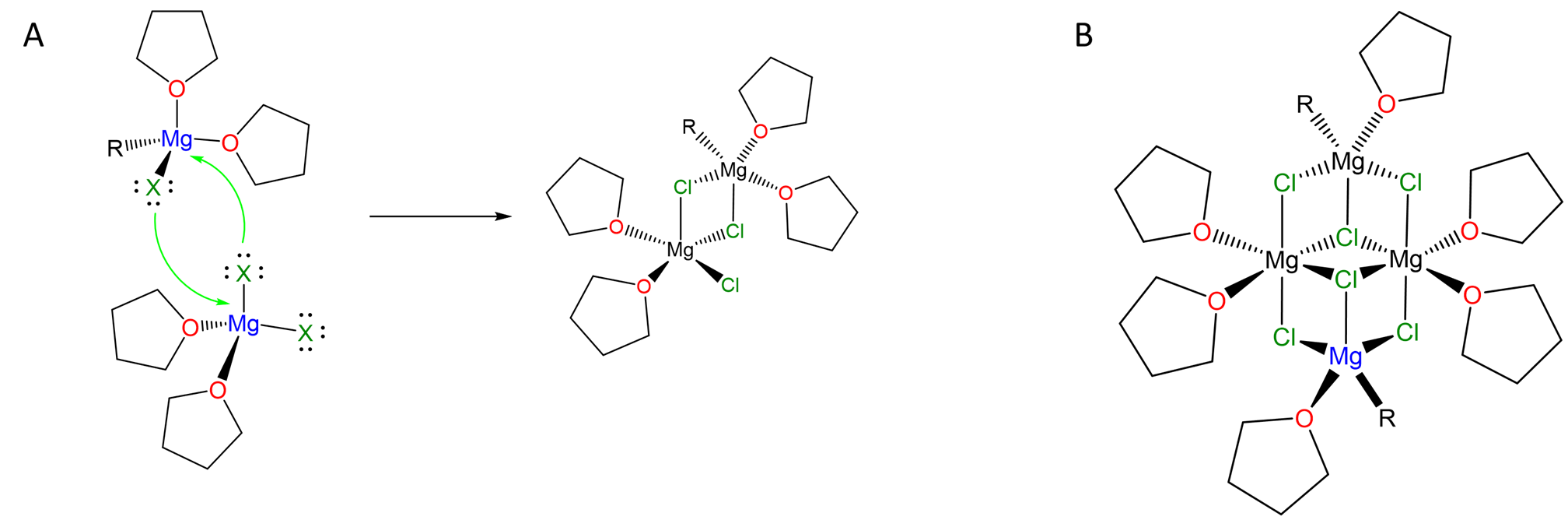
They also form clusters in which the halogen lone pairs are used to bridge multiple Mg centers, as illustrated by the complex in Scheme \(\sf{\PageIndex{III}}\).
Scheme \(\sf{\PageIndex{II}}\). (A) Halogens like Cl can bridge multiple metal centers via their lone pairs, allowing for the formation of species like (B) "(RMgCl)2(MgCl2)2". Redrawn from references 4 and 5.
The extent of covalency is even greater in the case of beryllium, which with a radius of only 113 Å and valence electrons that experience a Slater effective nuclear charge of +1.95 atomic charge units, has considerable ability to polarize nearby Lewis bases. As a result, beryllium tends to form polar covalent bonds rather than ionic ones.
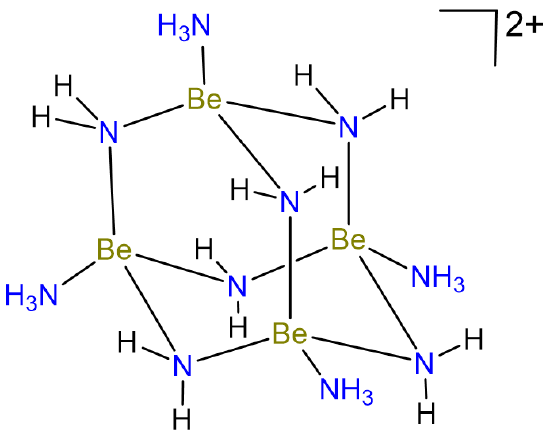
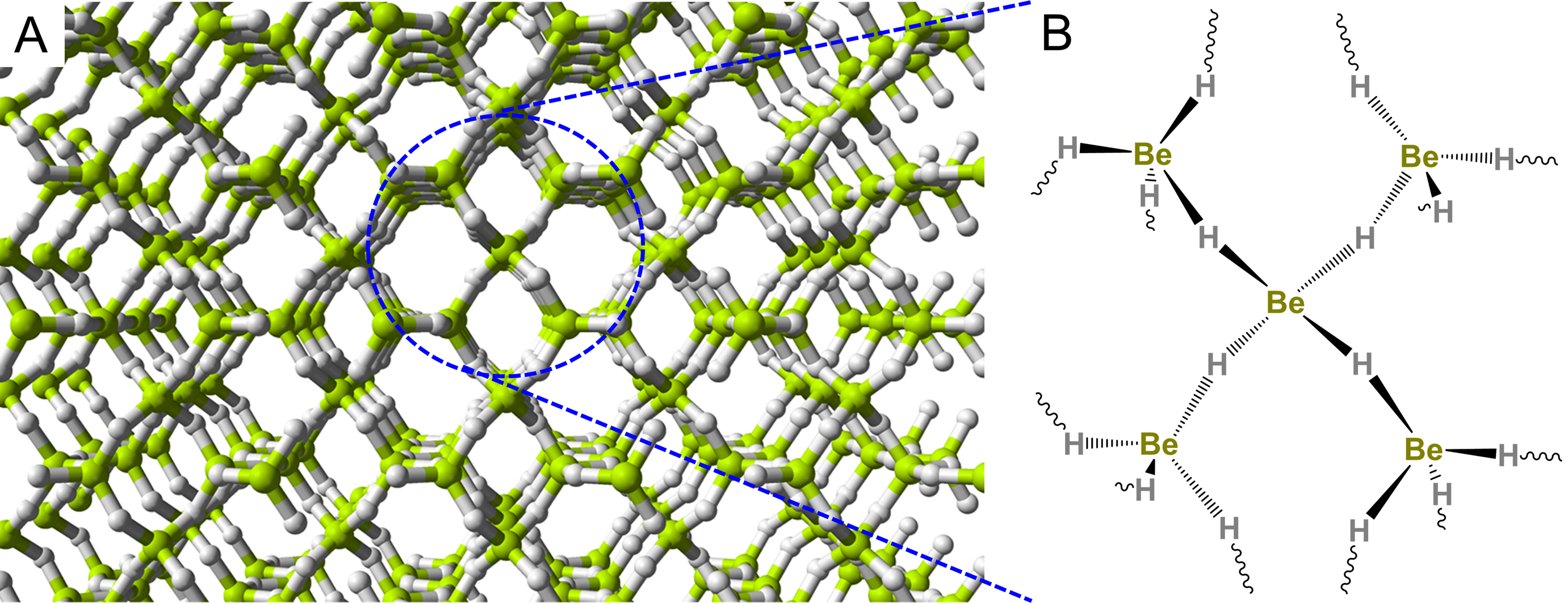
Because beryllium only possesses two valence electrons, its compounds also tend to be electron deficient and bridging Be-X-Be bonds are common. Thus in liquid ammonia, Be forms species with bridging Be-N-Be bonds like the tetrameric cluster shown in Scheme \(\sf{\PageIndex{III}}\).
Scheme \(\sf{\PageIndex{III}}\). Tetrameric Be cluster formed in liquid ammonia. Redrawn from reference 6.
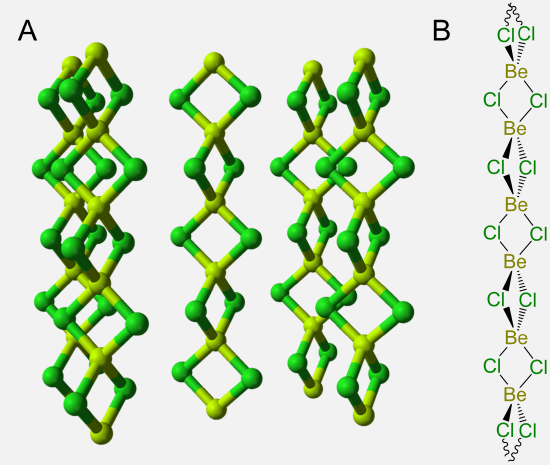
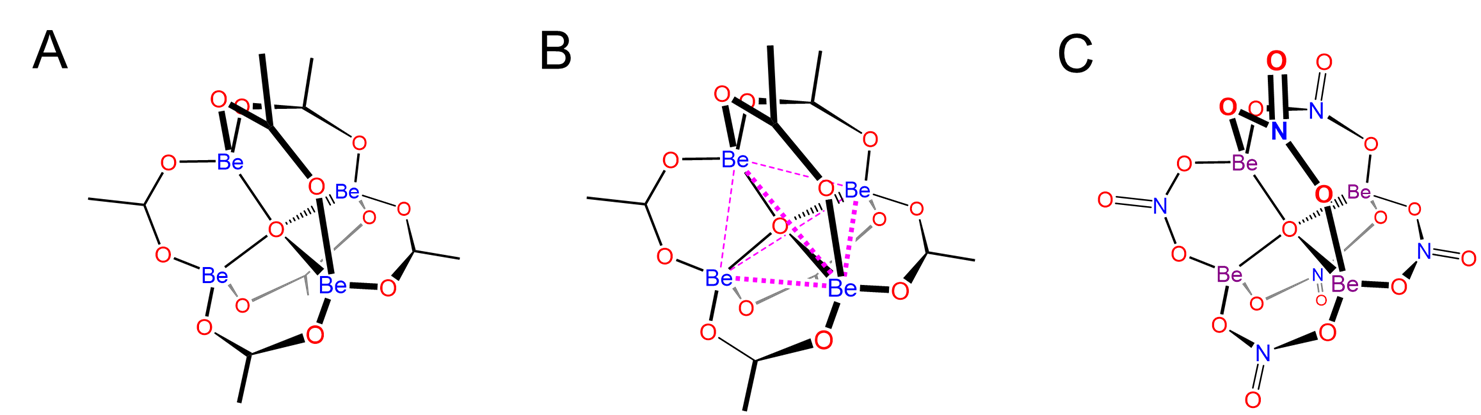
One particularly remarkable structure is that of basic beryllium acetate in which a central oxygen bridges four Be atoms, as shown in Scheme \(\sf{\PageIndex{IV}}\) along with that of Be4(NO3)6O, which is thought to possess an anlogous structure.
Scheme \(\sf{\PageIndex{IV}}\). (A) Structure of basic beryllium acetate, Be4(OAc)6O, (B) in which the central OBe4 tetrahedron is circumscribed to make it easier to see that the structure consists of a OBe46+ tetrahedron in which the Be---Be edges are linked by bridging acetate ligands. Zinc forms an analogous structure. (C) The structure of Be4(NO3)6O is postulated to be analogous to that of basic beryllium acetate, with bridging nitrate ligands taking the place of the acetates.
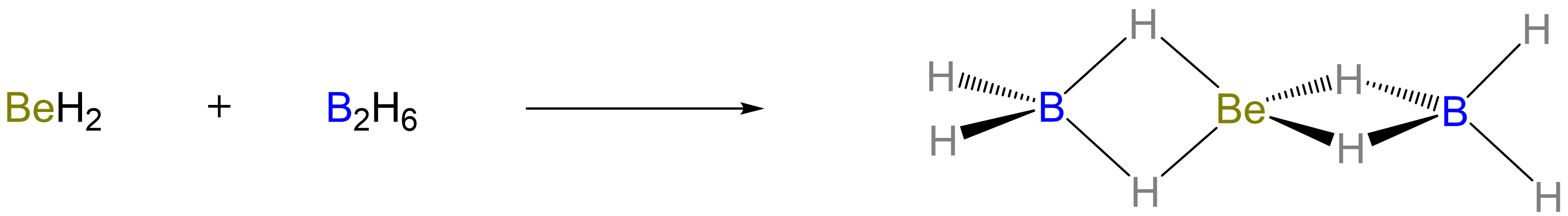
Beryllium hydride even forms an adduct with two BH3 to give a structure in which a central Be is linked to the terminal BH2 groups by 3-center-2-electron Be-H-B bonds, as shown in Scheme \(\sf{\PageIndex{V}}\).7
Scheme \(\sf{\PageIndex{V}}\). Formation of an adduct between BeH2 and two BH3 (in the form of B2H6).
References and Notes
1. Petrocelli, A.W. Superoxides. In Van Nostrand's Encyclopedia of Chemistry, G.D. Considine (Ed.). Wiley, 2005. doi:10.1002/0471740039.vec2421
2. The decomposition range midpoint for MgCO3, CaCO3, SrCO3, and BaCO3 are calculated from the data reported in Maitra, S., Chakrabarty, N., & Pramanik, J.. Cerâmica 2008, 54(331), 268-272.
3. The decomposition temperatures for BeSO4 , MgSO4 , CaSO4 , and SrSO4 are from Massey, A. G., Main group chemistry. E. Horwood: New York, 1990, pg. 152.
4. Seyferth, D., The Grignard Reagents. Organometallics 2009, 28 (6), 1598-1605.
5. Toney, J.; Stucky, G. D. J. Organomet. Chem. 1971, 28, 5.
6. Müller, M.; Karttunen, A. J.; Buchner, M. R., Speciation of Be2+ in acidic liquid ammonia and formation of tetra- and octanuclear beryllium amido clusters. Chemical Science 2020, 11 (21), 5415-5422.
7. The structure of BeB2H8 may also be thought of as an adduct between Be2+ and two BH4- ligands.
Contributors and Attributions
Stephen Contakes, Westmont College

