8.1.3: Ionization energy roughly increases towards the upper left of the periodic table but is also influenced by orbital energy and pairing energy effects
- Page ID
- 199661
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
As with electronegativity, the ionization energy of the main group elements (Figure \(\PageIndex{1}\)) decreases down a group and increases from left to right across the periodic table.
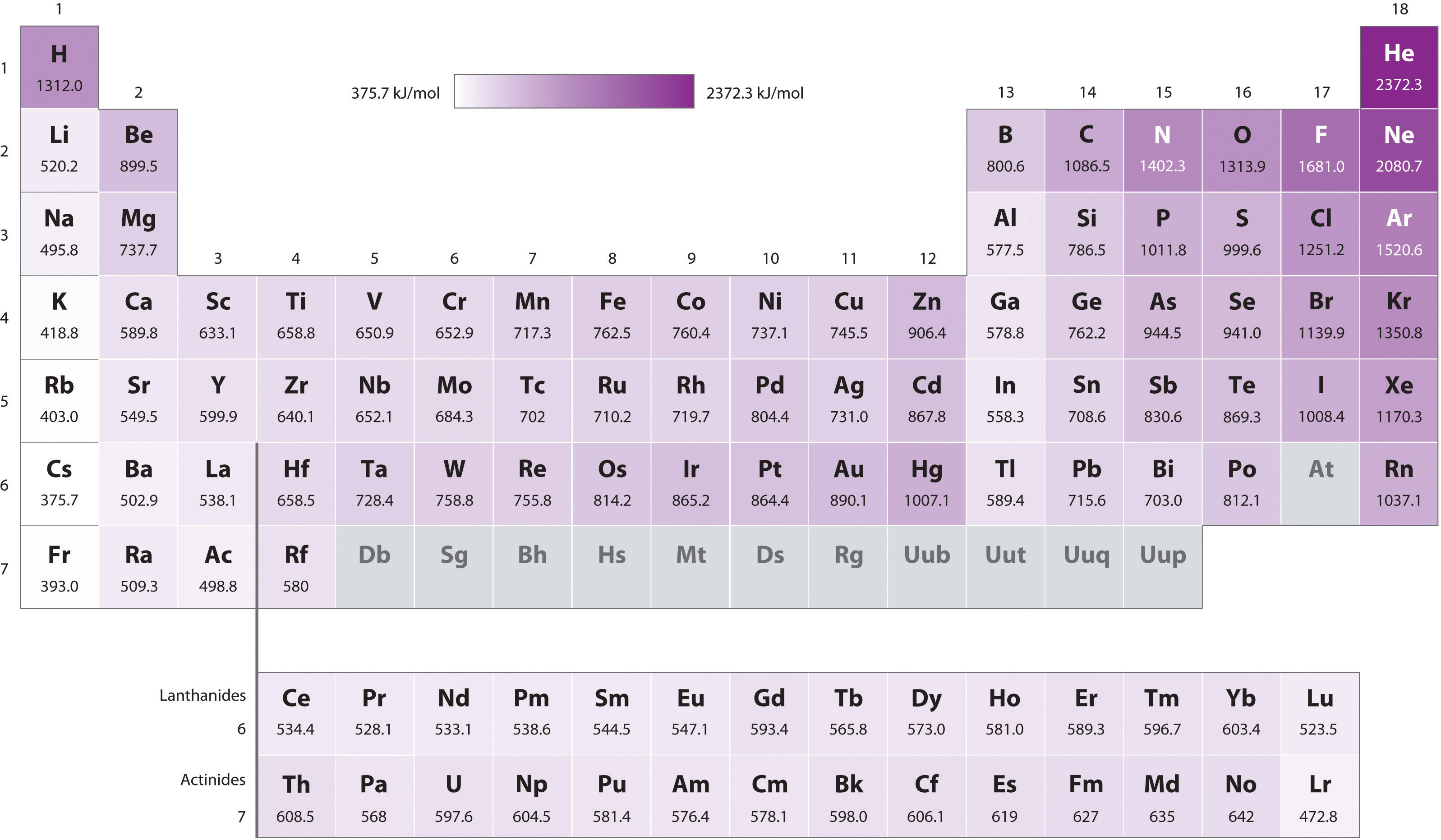
As may be seen in Figure \(\PageIndex{1}\), ionization energy does not increase steadily from left to right across a group. Instead, there are two discontinuities. These discontinuities are summarized in Figure \(\PageIndex{2}\).
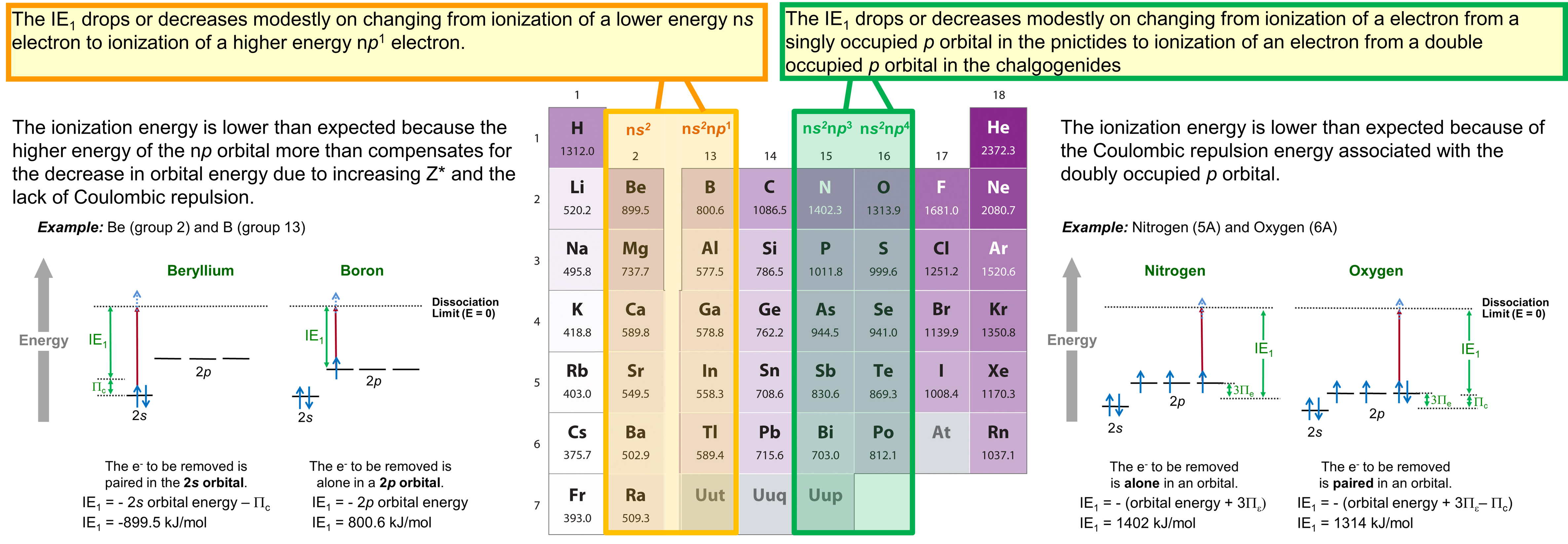
Briefly, the two discontinuities are as follows:
- There is a decrease or meager increase in ionization energy that occurs on going from the alkaline earth metals to the boron group, which is caused by the change in the highest occupied orbital from a lower energy ns to a higher energy np subshell.
- There is a decrease or anomalously modest increase in ionization energy on going from the pnictides to the chalcogenides caused by the presence of Coulombic repulsion in the chalcogenide np4 configuration that is not present in the pnictide np3 configuration.
These discontinuities illustrate how orbital energy and electron configuration effects should be considered when considering the reactivity of the elements.

