8.1.1.1: The metal-nonmetal-metalloid distinction and the metal-nonmetal "line" are useful for thinking about trends in elements' physical properties
- Page ID
- 199674
Two factors contribute to large-scale and rough variations in the sort of structure and bonding that main group elements engage in.
- Decreasing valence orbital energy and size on moving from the lower left to the upper right of the periodic table
- Increasing valence shell occupancy on moving from left to right across the main group
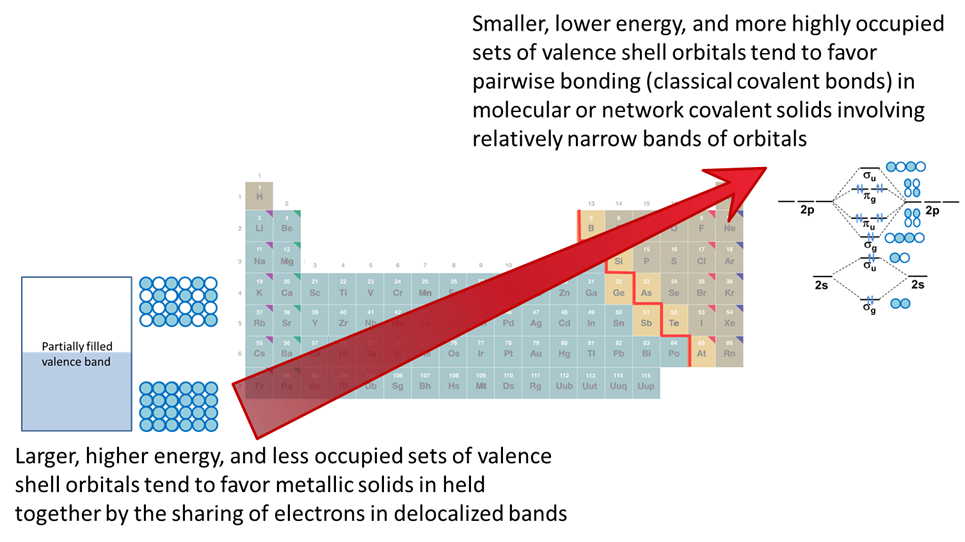
As summarized in Figure \(\PageIndex{1}\), the net effect of these trends is that
Elements in earlier groups with few valence electrons act as metals since they tend to either
- lose them to give cations with an empty valence shell
or
- share them by forming delocalized bonds in clusters and the solid state, although this tendency is less pronounced as one moves towards the top of the periodic table, since elements in earlier groups with more compact lower energy orbitals less readily ionize and more readily form strong covalent bonds (for example, consider the relative stability of alkyllithium and Mg-containing Gringard reagents compared to alkylsodium or calcium reagents)
Elements in later groups with many valence electrons act as nonmetals since they tend to either
- gain additional electrons to form anions with a stable filled valence shell
or
- form stable pairwise sigma bonds in molecular and network covalent solids, although this tendency is less pronounced as one moves down a group and the orbitals become more diffuse. As a result nonmetals exhibit an increased tendency to engage in delocalized and cluster bonding on moving down the periodic table.
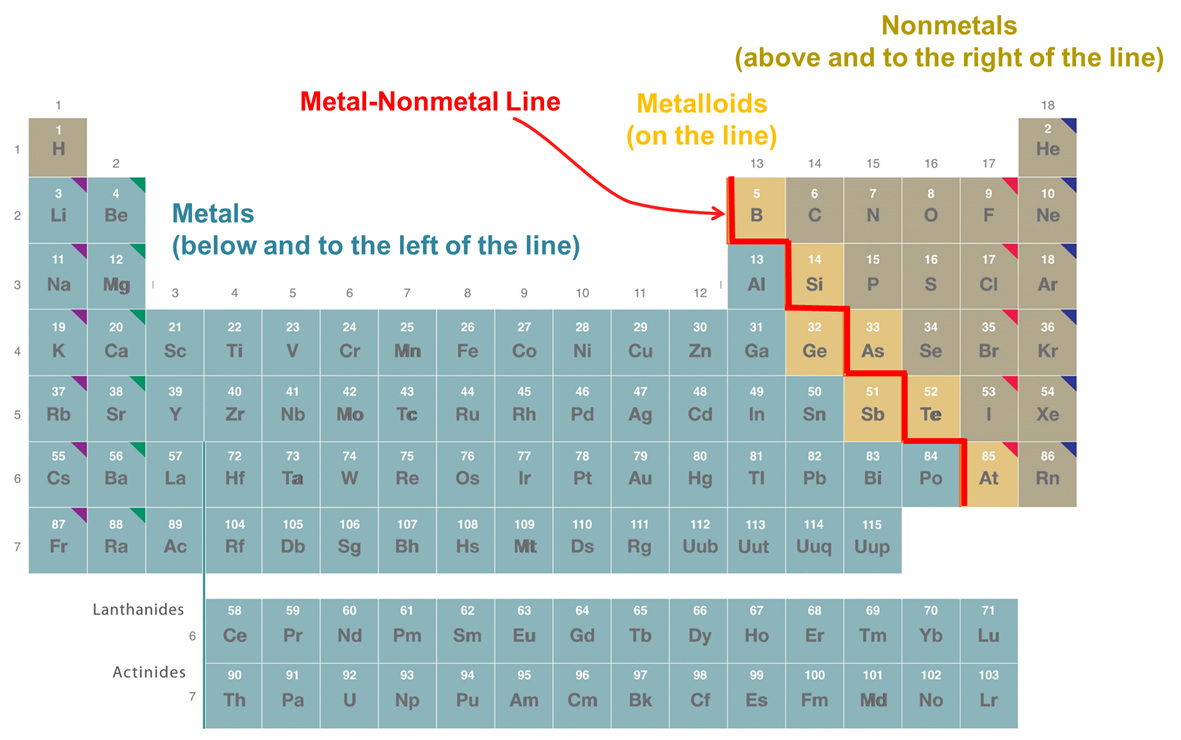
These periodic trends in bonding tendencies impact the sort of structures elements form and their physical properties. This is reflected in the classical division of the elements into metals, nonmentals, and metalloids depicted in Figure \(\PageIndex{2}\). As can be seen from Figure \(\PageIndex{2}\), the metals in the lower left of the periodic table are separated from the nonmetals in the upper right by the metal-nonmetal line. Most of the elements adjacent to the line (which ones are chosen varies between periodic tables) exhibit properties intermediate between metals and nonmetals and are called metalloids.
The metals towards lower left of the periodic table form metallic solids held together by delocalized bonding. As a result metals
- are malleable (able to be hammered into sheets) and ductile (able to be pulled into wires), since metals' solid state lattices may be deformed by dislocation movement (slip) without significantly disrupting the bonding.
- exhibit a high thermal conductivity and high electrical conductivity. Since they have an incompletely filled valence band delocalized throughout the structure, it is easy to transmit heat and charge by moving their electrons around.
In contrast, the nonmentals towards the upper right of the periodic table form molecular compounds and network covalent solids. In general these elements tend to
- be liquids, gases, or brittle solids. When present as solids, nonmetals tend to be brittle since deformation involves rupturing covalent bonds in the case of covalent solids or the rupture of intermolecular forces in the case of molecular solids.
- have low thermal and electrical conductivity, since for molecular solids the transfer of energy or electrons via collisions or through space is much much slower and, for network covalent solids held together by strong sigma bonds, involves excitation of electrons across a reasonably large band gap. Note that this is only a tendency. Network covalent solids held together by weaker covalent bonds or \( \pi\)-bonds sometimes have small or nonexistent band gaps. For example, the graphite allotrope of carbon is an outstanding electrical conductor because its \(\pi\)-valence band and \(\pi\)*-conduction band overlap, leaving no band gap.
The metalloids along the metal-nonmetal line form solids that exhibit bonding patterns and properties intermediate between those of metals and nonmetals. This is because is in reality there is a graduation in element properties as one moves from the lower left to the upper right of the periodic table. Consequently, although the distinction between metals, nonmetals, and metalloids can be a helpful one, these categories should not be understood too rigidly.
The same is true of the tendency of metals and nonmetals to form ionic compounds with one another, owing to the tendency of metals to lose electrons and nonmetals to gain them. In practice, there is a graduation in ionic character based on the difference in the elements' electronegativites and the size/polarizability of the atoms involved. As a result many compounds between metals and nonmetals may be profitably thought of as involving polar covalent bonds.
Contributors and Attributions
Stephen M. Contakes (Westmont College)

