24.16.2: Organomercury Chemistry
- Page ID
- 85474
In this lecture you will learn the following
- Dialkymercury preparation.
- Mercury toxicity.
- Mercury poisoning.
Organometallic compounds of mercury:
\[2 \mathrm{RMgX}+\mathrm{HgX}_2 \longrightarrow \mathrm{HgR}_2+\mathrm{MgX}_2\]
Reaction proceeds due to both electronegativity and hardness considerations.
Dialkylmercury compounds are very versatile starting materials for the synthesis of many organometallic compounds of more elctropositive metals by transmetallation. However, owing to high toxicity of alkylmercury compounds, other synthons are preferred. In striking contrast to the high sensitivity of dimethylzinc to oxygen, dimethylmercury survives exposure to air.
Mercury Toxicity
The toxicity of mercury arises from the very high affinity of the soft Hg atom for sulfhydryl (—SH) groups in enzymes. Simple mercury-sulfur compounds have been studied as potential analogs of natural systems. The Hg atoms are most commonly four-coordinated, as in [Hg2(SMe)6]2-.
Mercury poisoning was a serious concern even from early days. Issac Newton, Alfred Stock worked in the early 20th century. Later in 60s awareness came following the incidence of brain damage and death it caused among the inhabitants in Minamata, Japan. Mercury from a plastic company was allowed to escape into a bay where it found its way into fish that were later eaten. Research has shown that bacteria found in sediments are capable of methylating mercury, and that species such HgMe2 and [HgCH3]+ enter the food chain because they readily penetrate cell walls. The bacteria appear to produce HgMe2 as a means of eliminating toxic mercury ions through their cell walls and into the environment.
References
- Inorganic Chemistry, Principles of structure and reactivity, 4th edition; 1993, J. E. Huheey, E. A. Keiter, R. L. Keiter, Addison-Wesley Publishing Co, New York.
- Advanced Inorganic Chemistry, 6th edition, 1999, F. A. Cotton, G. Wilkinson, C. A. Murillo, M. Bochmann, John Wiley and Sons, New York.
- Organometallics, A Concise Introduction, 2nd edition (revised), 1992, Ch. Elschenbroich, A. Salzer, Weinheim, Germany.
- Inorganic Chemistry, 3rd Edition, 1999, D. F. Shriver, P. W. Atkins, Oxford University Press, Oxford.
- Inorganic Chemistry, 2nd Edition, 2005, C.C. Housecroft and A. G. Sharpe, Pearson, Prentice Hall, England.
Methylmercury
The usual method of ingestion of a metal into the body is:
a) Orally - the Gastrointestinal Tract
b) via the lungs - the Respiratory Tract
a) Orally - (mouth, stomach, small intestine): Food digestion begins in the mouth where saliva containing the enzyme amylase beaks down starch to lower sugars. Most digestion occurs in the stomach in the presence of HCl (pH ~1.6 ie. 0.17M HCl). In the case of mercury it is quite readily absorbed through the stomach since reaction of mercury salts with HCl produces HgCl2. This neutral covalent molecule (solubility in H2O 0.5 g/100 mL but 8g/100 mL in ethanol) is absorbed far better than most inorganic ions and this no doubt contributes to its high toxicity.
b) Respiratory tract (nose, lungs): This is usually unimportant for most metals but in some cases it can be more efficient that via the gastrointestinal tract. For example, lead where 50% of Pb in air can be absorbed but only 5-10% via the gastrointestinal tract. Volatile dimethyl mercury is another case for concern.
dimethylmercury
The Mercury Cycle
Mercury is 62nd in terms of natural abundance and is found everywhere, usually as the mineral cinnabar, HgS, although 30 minerals containing Hg are known. The oxidation states are Hg(0), Hg(I) and Hg(II), where Hg(I) has been shown to exist as Hg22+.
HgS - cinnabar
The three Hg species are related by the disproportionation:
Hg22+ → Hg0 + Hg2+ E° = -0.131 V or K= 6 x 10-3
In addition:
Hg22+/Hg E° = 0.789 V
Hg2+/Hg E° = 0.854 V
This means that to oxidise Hg to Hg22+ an oxidising agent with potential > 0.789V is required, but very importantly < 0.854 V, otherwise oxidation to Hg2+ will occur. There are no common oxidants that fit this arrangement so if any reaction occurs the product will be Hg2+. The equilibrium constant of 6 x 10-3 shows that when Hg(I) is formed it is moderately stable, however any agent that reduces the Hg(II) concentration automatically drives the reaction from Left → Right. Given that many Hg(II) derivatives are insoluble then this clearly restricts the range of Hg(I) compounds.
Minamata Disease
There have been several serious outbreaks of mercury poisoning. The most famous was between 1953 and 1965 at Minamata Bay in Japan when 46 people died and 120 suffered severe symptoms. As of March 2001, 2,265 victims had been officially recognised (1,784 of whom had died) and over 10,000 had received financial compensation from Chisso. By 2004, Chisso Corporation had paid $86 million in compensation, and in the same year was ordered to clean up its contamination. On March 29th, 2010, a settlement was reached to compensate as-yet uncertified victims.
The disease was first noticed in cats (who were seen throwing themselves into the sea) and was quickly traced to mercury poisoning acquired as a result of eating contaminated fish (5-10 ppm Hg). The investigations that followed showed that the fish had acquired the high mercury due to the dumping of inorganic mercury salts and methylmercury from the Chisso Co. plastics factory upstream.
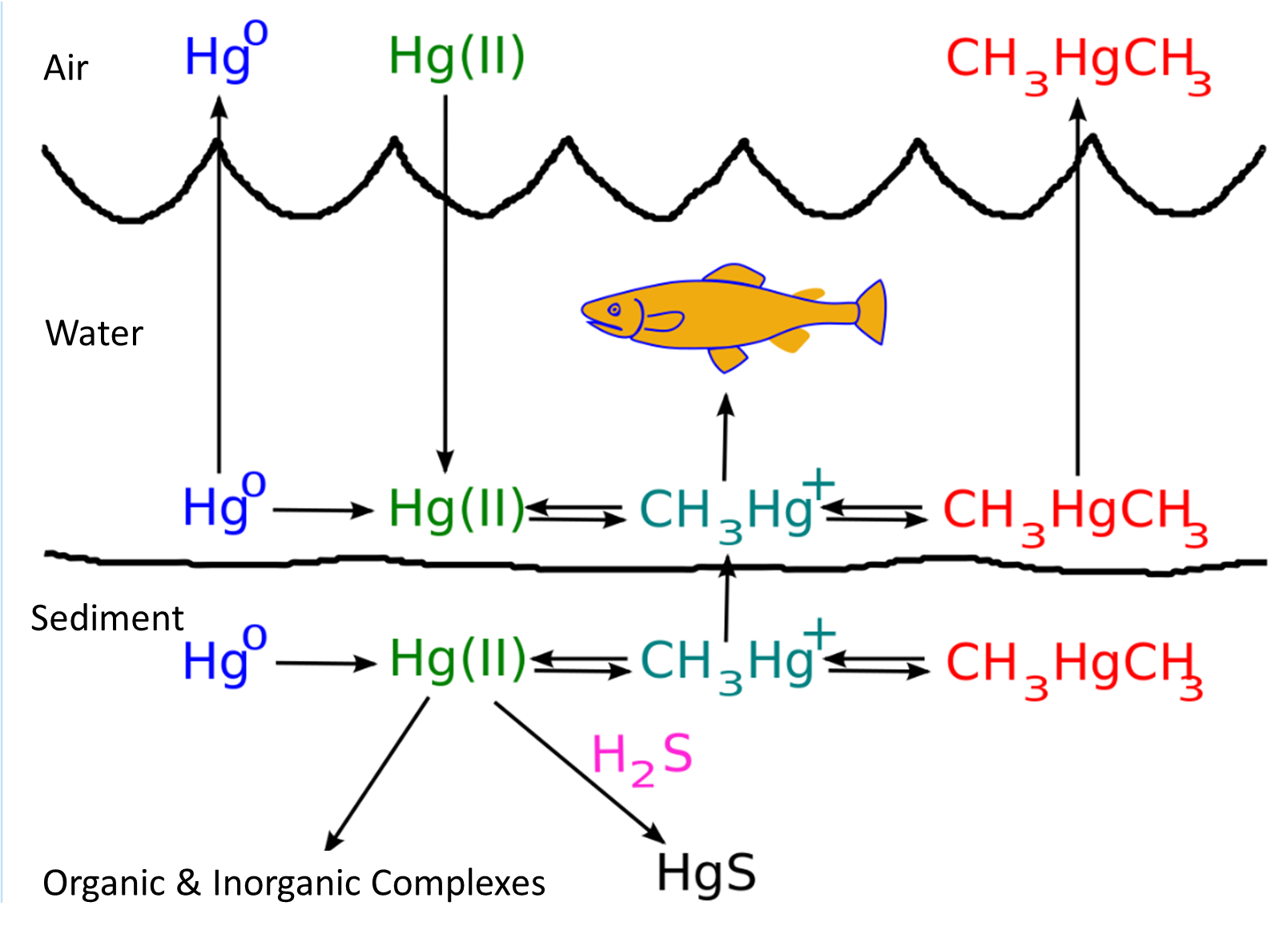
Analysis of fish exhibits from museums, some over 90 years old, has shown that mercury levels for ocean fish are similar but that river fish levels have risen as a result of man-made contamination. The forms of mercury occuring in the environment are Hg2+ and methylmercury, either MeHg+ or Me2Hg. Interconversion can be affected by microorganisms.
Aerobes can solubilize Hg2+ from cinnabar (Ksp ~10-53) which in sediments was considered safe since the solubility product was so small. The conversion of S2- → SO32- → SO42- allows the insoluble sulfide to breakdown and in the process other Hg(II) salts are formed or the mercury may get reduced to Hg(0) enzymatically.
\[\ce{Hg^{2+} + NADH + H^{+} → Hg^{0} + NAD^{+} + 2 H^{+}}\]
where NADH = reduced form of nicotinamideadeninedinucleotide. This conversion can be considered as a detoxification process since Hg0 is more easily eliminated.
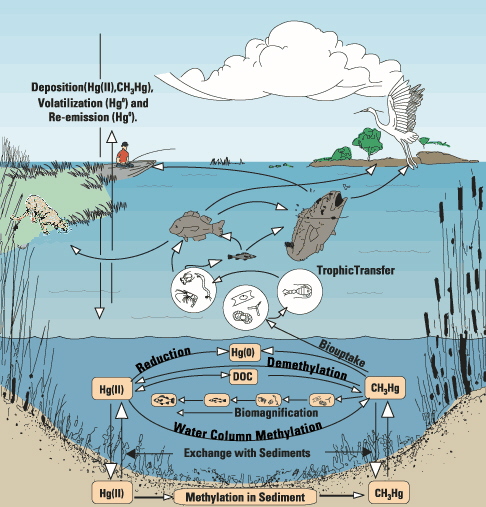
In the environment, sulfate-reducing bacteria take up mercury in its inorganic form and through metabolic processes convert it to methylmercury. Sulfate-reducing bacteria are found in anaerobic conditions, typical of the well-buried muddy sediments of rivers, lakes, and oceans where methylmercury concentrations tend to be highest. Sulfate-reducing bacteria use sulfur rather than oxygen as their cellular energy-driving system. One hypothesis is that the uptake of inorganic mercury by sulfate-reducing bacteria occurs via passive diffusion of the dissolved complex HgS. Once the bacterium has taken up this complex, it utilizes detoxification enzymes to strip the sulfur group from the complex and replaces it with a methyl group:
\[\ce{HgS → CH3Hg(II)X + H2S }\]
Upon methylation, the sulfate-reducing bacteria transport the new mercury complex back to the aquatic environment, where it is taken up by other microorganisms. Bacteria eliminate Hg by methylating it first to MeHg+ and Me2Hg. The detoxification process for them is the reverse for us unfortunately! The conversion probably involves vitamin B12 a methyl-cobalt organometallic compound so this is another example of synthesis involving transmetallation.
The major source of methylmercury exposure in humans is consumption of fish, marine mammals, and crustaceans. Once inside the human body, roughly 95% of the fish-derived methylmercury is absorbed from the gastrointestinal tract and distributed throughout the body. Uptake and accumulation of methylmercury is rapid due to the formation of methylmercury-cysteine complexes. Methylmercury is believed to cause toxicity by binding the sulfhydryl groups at the active centers of critical enzymes and structural proteins. Binding of methylmercury to these moieties constitutively alters the structure of the protein, inactivating or significantly lowering its functional capabilities.
Once the Me2Hg is formed it is volatile and when released into the atmosphere it is readily photolysed by UV light
\[\ce{Me2Hg → Hg^{0} + 2 CH3^{\cdot} → CH4 \,text{ or } C2H6}\]
Other microorganisms can convert MeHg+ to Hg0 + CH4 that is make the mercury considerably less toxic to humans.
Summary
Organic mercury tends to increase up the food chain, particularly in lakes. The mud at the bottom of a lake may have 100 or 1000 times the amount of mercury than is in the water. Bacteria, worms and insects in the mud extract and concentrate the organic mercury. Small fish that eat them further concentrate the mercury in their bodies. This concentration process, known as "bioaccumulation", continues as larger fish eat smaller fish until the top predator fish in the lake may have methylmercury levels in their tissues that are up to 1,000,000 times the level in the water in which they live. We then eat the fish....
To consume a human being would be extremely unhealthy for any animal. Humans carry the highest concentration of toxic chemicals of all creatures on the planet. Their livers, hearts, kidneys and brains are so heavily contaminated with hundreds of different synthetic chemicals that if humans were slaughtered as a meat source, they'd never pass USDA food safety standards.
Table \(\PageIndex{1}\): Properties of Me2Hg
| Molecular formula | C2H6Hg |
| Molar mass | 230.66 g mol-1 |
| Appearance | Colourless liquid |
| Density | 2.96 g/mL |
| Melting point | -43 °C |
| Boiling point | 87-97 °C |
| Solubility in water | Insoluble |
Laboratory Preparation:
Hg + 2 Na + 2 CH3I → (CH3)2Hg + 2 NaI
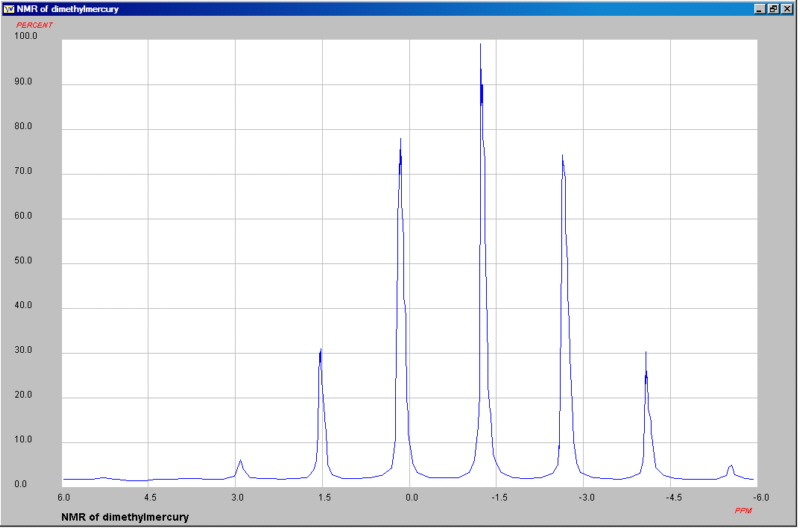
1H NMR of dimethylmercury showing 199Hg coupling (J ~100.9 Hz)
199Hg has a nuclear spin of ½ and natural abundance of 16.87%. Can you explain the observed splitting pattern?
Methylmercury
Dimethylmercury
Prof Wetterhahn -mercury

