23.4B: Aluminium
- Page ID
- 34551
In this lecture you will learn the following
- Preparation and reactivity of organoboron and organo aluminium compounds.
- Influence of Lewis acidity on structural features.
Organoaluminium compounds
With less bulky alkyl groups, dimerization occurs and one of the distinguishing features of alkyl bridge is the small Al-C-Al angle, which is ~ 75°.
The 3c,2e bonds are very weak and tend to dissociate in the pure liquid which increases with increase in the bulkiness of the alkyl group.

![]()
Perpendicular orientation of pheynl groups in Al2Ph6
Triphenylaluminium exists as a dimer with bridging η1-phenyl groups lying in a plane perpendicular to the line joining the two Al atoms.

This structure is favored partly on steric grounds and partly by supplementation of the Al-C-Al bond by electron donation from the phenyl π-orbitals to the Al atoms.
Tendency for bridging: X > Ph > alkyl
3c,2e bonds formed by a symmetric combination of Al and C orbitals

An additional interaction between the pπ orbital on C and an antisymmetric combination of Al orbitals.

Synthesis
Very useful as alkene polymerization catalysts and chemical intermediates.
Expensive carbanion reagents for the replacement of halogens organic groups by metathesis.
Laboratory scale preparations involves:
![]()
Commercial method:
![]()
Commercial method for ethylaluminium and higher homologs:
![]()
The reaction probably proceeds by the formation of a surface Al—H species that adds across the double bond of the alkene in a hydrometallation reaction.

Reactions:
Alkylaluminum compounds are mild Lewis acids and form complexes with ethers, amines and anions. When heated, often β-hydrogen elimination is responsible for the decomposition of ethyl and higher alkylaluminium compounds. E.g. Al(iC4H9)3
Tendency towards bridging structure is: PR2-> X -> H -> Ph-> R-.
The first organoaluminium compound, an alkylaluminium sesqui-halide, Et3Al2I3 was reported in 1859 and was formed by reaction of elemental Al and EtI. "Me3Al" was obtained by George Buckton from aluminium and dimethylmercury as early as 1865. In hydrocarbon solution and in the solid state there is a tendency for R3Al to dimerise; this is very dependent on the size of the R group, eg a dimer for Me but monomer for tert-butyl. Me(t-Bu)5Al2 is found to be a dimer as well with the methyl group and 1 of the t-Bu groups in the bridging positions. The bridging ability is found to be Me > Et > t-Bu and clearly the case above is not what would be predicted on purely statistical terms since there are 5 times as many t-Bu groups as Me groups and there are twice as many terminal positions as bridging positions so the methyl group might have been expected to fill a terminal position.
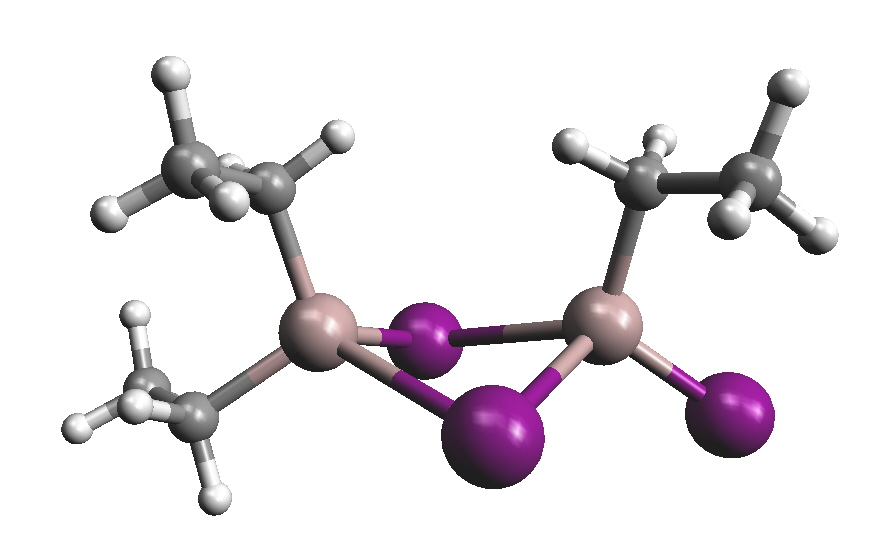
Et3Al2I3
Many organoaluminium compounds are commercially available at quite reasonable prices so that is is rarely necessary to have to prepare them in the laboratory. Reaction of Al turnings with organic halides leads to the alkylaluminium sesqui-halides. The reaction is very exothermic. The sesqui-halides do not have sharp melting or boiling points because they are in fact equilibrium mixtures:
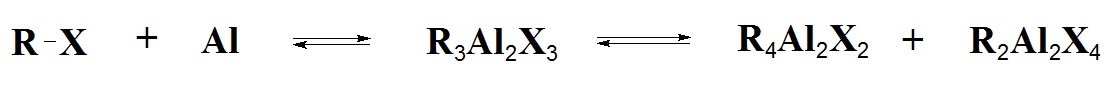
R3Al2X3 sesqui halide equilibria
It took almost 100 years before K. Ziegler discovered the synthetic and catalytic potential of organoaluminum compounds. From an industrial viewpoint, the organic compounds of aluminium are probably the most important organometallic compounds.
The reasons for this include:
- inexpensive synthesis from olefin + H2 + Al (pressure).
- R3Al can dimerise olefins
- higher alcohols (→ detergents) are made from R3Al + CH2=CH2.
- In combination with Ti and Zr compounds, R3Al can polymerise ethylene to give polyethylene
- Reactions with R3Al proceed in hydrocarbons or even without solvent (unlike RMgX !).
- Useful commercially for polymer synthesis catalysts
Karl Ziegler, who pioneered basic research in the field of organoaluminium compounds developed a strikingly simple yet versatile process for the synthesis of organoaluminium compounds from inexpensive starting materials. The Ziegler Direct Process allows the synthesis of triethylaluminium from aluminum metal, hydrogen and ethylene. The process involves the following:
2Al + 3H2 + 6RHC=CH2 → Al2(CH2CH2R)6 {R=H for (Et3Al)2}
The Ziegler-Natta polymerisation catalysts were originally formed from Et3Al with TiCl4 and a schematic representation of the reactions at the heterogeneous surface is given below:
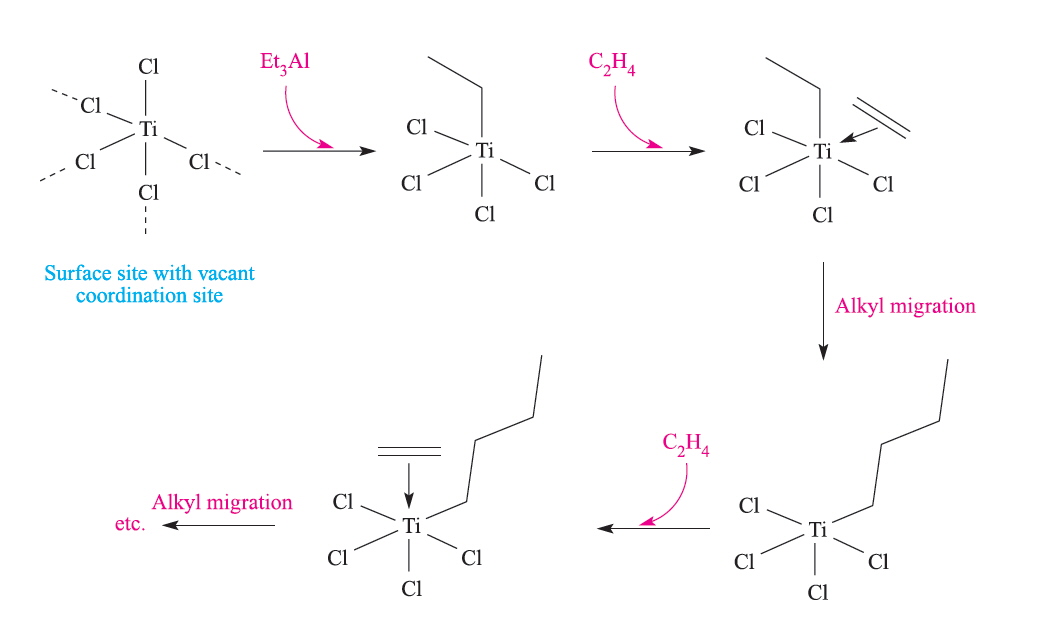
Ziegler Natta polymerisation
Polymerisation of ethene to high-molecular mass polyethylene occurred at relatively low pressures and the polymers were stereoregular. What this means is that isotactic polymers are formed where the R groups are all located on the same side of the carbon backbone. The resulting product gives a crystalline material since packing is more regular. The other varieties of linear polymer are called syndiotactic and atactic and in the first the R groups are on alternate sides of the carbon backbone and in the latter they are randomly distributed.
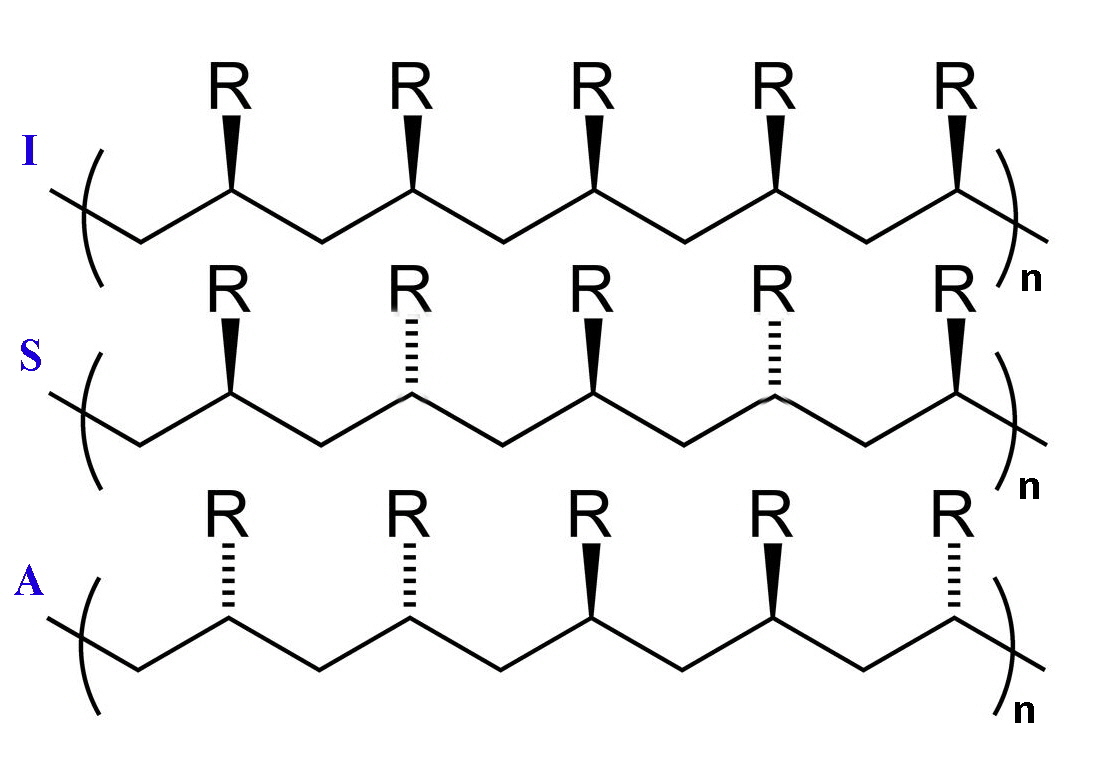
Isotactic, syndiotactic and atactic linear polymer chains
The importance of his research was soon recognized and he received the 1963 Nobel Prize for Chemistry, together with Giulio Natta who made fundamental contributions to the polymerization field.
Compounds of the type Me2Al(μ-Ph)2AlMe2 and Ph2Al(μ-Ph)2AlPh2 show that the bridging phenyl groups are almost vertical compared to the R2Al-AlR2 plane and the ipso-carbon is a distorted tetrahedral.
A similar case is found with bridging alkynes -C≡C-R where the terminal groups are t-Bu and when the Al is changed to Ga. Although in the latter case the phenyl groups are at right angles to each other.
However a very different arrangement with bridging alkynes has been found where they point towards one of the Al centres. Here the bonding is interpreted as a mixture of σ and π bonds; an Al-C σ bond connects to the first Al but the second Al centre is bound using the C≡C π bond. Examples of this are for Me2Al(PhC≡C)2AlMe2 and for the analogous Gallium compound.
Problems
- Propose a structure for Al2(Me)4Cl2.
Solution:
Similar to diborane:
- Solution:
Lower force constant for Si-O-Si bending. - Explain how the difference in reactivity between Al-C and Si-C bonds with O-H groups leads to the choice of different strategies for the synthesis of aluminum and silicon alkoxides.
Solution:
For reaction of Al2Me6 with alcohols, see the text book by Shriver and Atkins.Al2Me6 + 6 MeOH → 2Al(OMe)3 + 6 CH4
Tetramethylsilane does not react with methyl alcohol. Therefore, the appropriate reagent is tetrachlorosilane and the reaction is:SiCl4 + 4 MeOH → Si(OMe)4 + 4HCl.
Problems: (contd..)
- Compare formulas of the most stable hydrogen compounds of germanium and arsenic with those of their methyl compounds. Can the differences be explained in terms of the relative electronegativities of C and H?
Solution:
GeH4, GeR4; AsH3, AsR3.
The stability of hydrides and alkyls are very similar for each element. This may due to similar H and C electronegativity.
- To buy from a chemical company, the price of trimethylaluminum is higher than that of triethylaluminum. Is it due to the methods of synthesis? Rationalize the price difference.
Solution:
Triethylaluminum can be made in larger quantities by direct reaction of aluminum, hydrogen gas and ethane gas which is a cheaper method.
![]()
Preparation of trimethyaluminum involves a more expensive route such as MeCl and aluminum to form Al2Me4Cl2 followed by treatment with sodium metal. The sodium metal and MeCl are not cheap as compared to ethane and hydrogen gases.
 |
Contributors and Attributions
Prof. Robert J. Lancashire (The Department of Chemistry, University of the West Indies)
- http://nptel.ac.in/courses/104101006/8

