23.4A: Boron
- Page ID
- 34550
Organoboron Compounds
BMe3 is colorless, gaseous ( b.p. -22 °C), and is monomeric. It is pyrophoric but not rapidly hydrolyzed by water.
Alkylboranes can be synthesized by metathesis between BX3 and organometallic compounds of metals with low electronegativity, such as RMgX or AlR3.
![]()
Why dibutyl ether as a solvent: Has much lower vapor pressure than BMe3 and as a result the separation by trap-to-trap distillation on a vacuum line is easy.
Also, there is a very weak association between BMe3 and OBu2(Me3B:OBu2).
Although, trialkyl- and triarylboron compounds are mild Lewis acids, strong carbanion reagents lead to anions of the type [BR4]-.
Example, Na[BPh]4: The bulky anion hydrolyses very slowly in neutral or basic water and is useful for the preparation of large positive cations.
![]()
K[BPh]4 is insoluble, used for the gravimetric estimation (determination) of potassium, an example of the low solubility of large-cation and large-anion salts in water.

Organohaloboron compounds are more reactive than simple trialkylboron compounds.
Preparation

Reactions: (Protolysis reactions with ROH, R2NH and other reagents)
![]()
Boron
Most alkyl and aryl organoboron compounds are reasonably stable in water, although they may still be fairly air sensitive even pyrophoric. They are usually monomeric. For example, triethylborane (TEB) is strongly pyrophoric, igniting spontaneously in air. It burns intensely with a very hot flame. The color of the flame is apple-green, which is characteristic for boron compounds. Its vapours may cause flash fires. This was first noted by Frankland in 1860 when he first prepared Et3B from Et2Zn by transmetallation.
Et3B is soluble in tetrahydrofuran and hexane, and is not pyrophoric when in solution. However the solution can slowly react with atmospheric moisture. If the TEB solutions are exposed to air for prolonged time, unstable organic peroxides may form. It has been found to be toxic to the peripheral nervous system, kidneys and testes and is extremely corrosive.
The Lockheed SR-71 strategic reconnaissance aircraft uses as fuel a mixture of hydrocarbons known as JP-7. The very low volatility and relative unwillingness of JP-7 to be ignited required a pyrophoric material like triethylborane (TEB) to be injected into the engine in order to initiate combustion and allow afterburner operation in flight.

Lockheed SR-71, 'Blackbird'
JP-7 jet fuel was designed to have a relatively high flash point (60 °C) to cope with the heat. In fact, the fuel was used as a coolant and hydraulic fluid in the aircraft before being burned. The fuel also contained fluorocarbons to increase its lubricity, an oxidizing agent to enable it to burn in the engines, and even a caesium compound, A-50, to help disguise the exhaust's radar signature.
JP-7 is very slippery and extremely difficult to light in any conventional way. The slipperiness was a disadvantage on the ground, because inevitably the aircraft leaked small amounts of fuel when not flying, fortunately JP-7 was not a fire hazard. When the engines of the aircraft were started, puffs of triethylborane (TEB), which ignites on contact with air, were injected into the engines to produce temperatures high enough to ignite the JP-7 initially. The TEB produced a characteristic puff of greenish flame that could often be seen as the engines were ignited. TEB was also used to ignite the afterburners. The aircraft had only 600 ml of TEB on board for each engine, enough for at least 16 injections (a counter advised the pilot of the number of TEB injections remaining), but this was considered more than enough for the requirements of any missions it was likely to carry out.
The triarylborane, BPh3, is less reactive and forms the salt Na[BPh4] that is water soluble and is useful as a precipitating agent for large metal ions.
R2BCl and RBCl2 have been prepared by transmetallation and these have been used to generate species like R2B(μ-H)2BR2. One example of this is the reagent (9-BBN) that is used for the regioselective reduction of ketone, aldehydes, alkynes and nitriles. Its highly stereoselective addition on olefins allows the preparation of terminal alcohols by subsequent oxidative cleavage with H2O2 in aq. KOH. The steric demand of 9-BBN greatly suppresses the formation of the 2-substituted isomer compared to the use of borane.
It has been found to be a useful reagent for the Suziki Reaction:
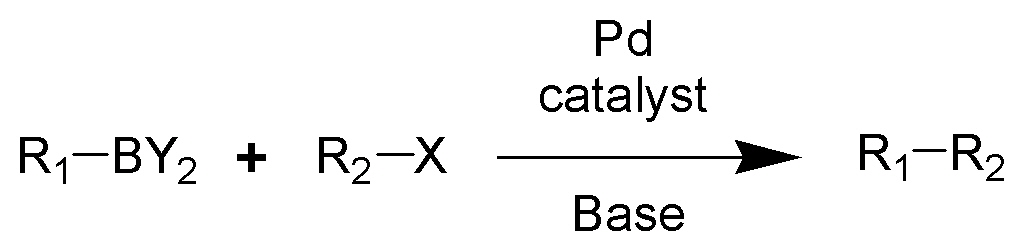
Suzuki Reaction Scheme
Hydroboration
The hydroboration-oxidation reaction is a two-step organic chemical reaction that converts an alkene into a neutral alcohol by the net addition of water across the double bond. The hydrogen and hydroxyl group are added in a syn addition leading to cis stereochemistry. Hydroboration-oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon. The reaction was first reported by Herbert C. Brown in the late 1950s and he received the Nobel Prize in Chemistry in 1979. Shown below is the original reaction described in 1957 where hex-1-ene is converted to hexanol.
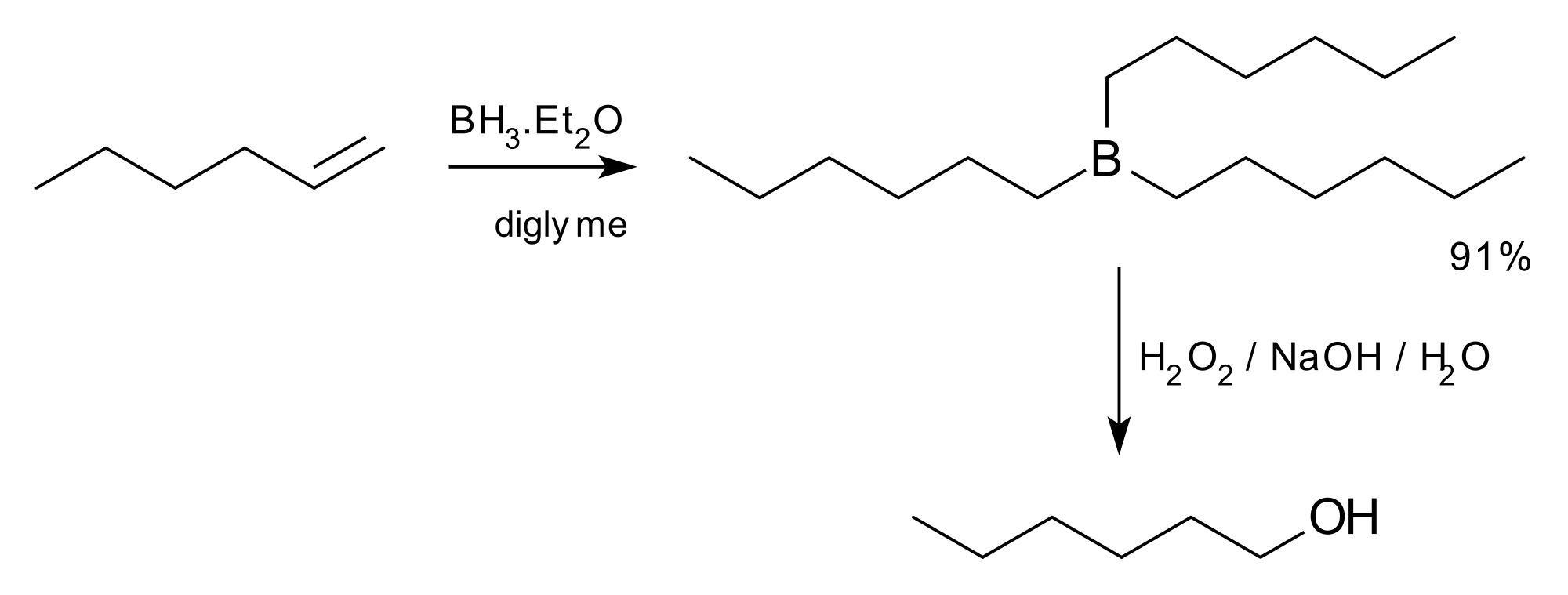
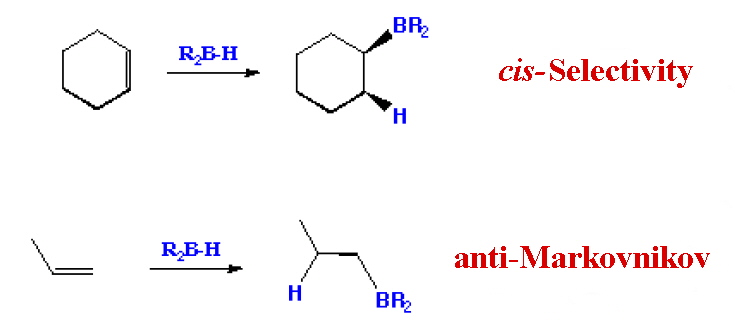
H. C. Brown reaction of 1957 other hydroboration reactions
Diborene
The first structurally characterized neutral diborene containing a B=B double bond was reported in 2007. It was prepared by the following scheme:
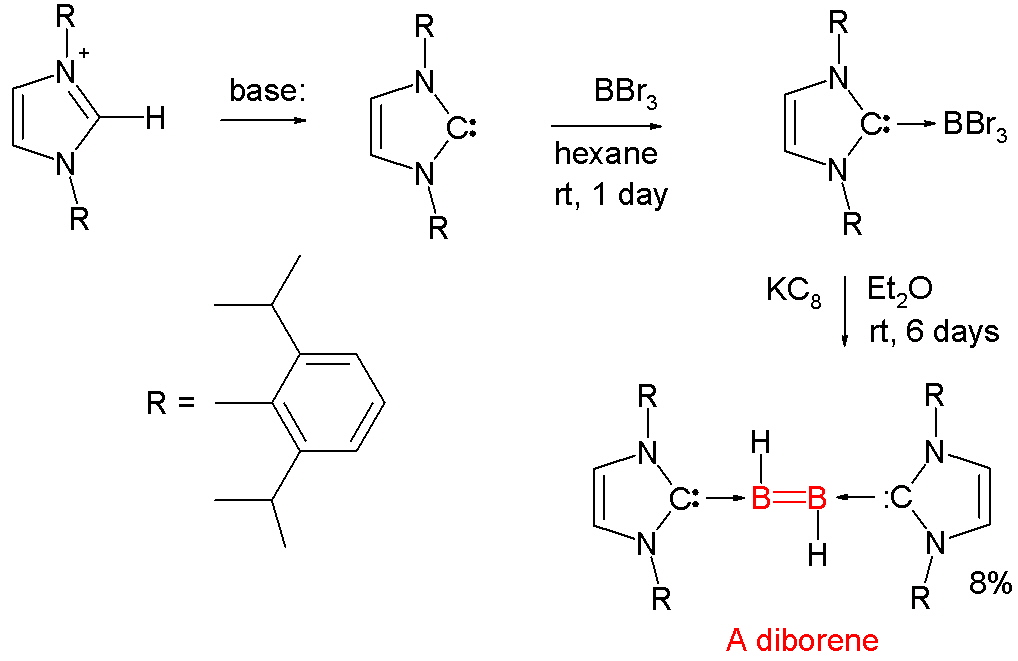
Synthesis of a diborene
According to the authors, the carbene ligands were the key to the ability of boron to form the diborene's B=B bond. The divalent carbon atom of each carbene is able to donate its two free electrons to form a carbon-boron bond, allowing the boron's three valence electrons to form a bond to hydrogen and a σ and a π bond to the other boron.

