10.7D: Molecular Hydrides and Complexes Derived from them
- Page ID
- 34069
Molecular hydrides - covalent hydrides and organic compounds
Hydrogen forms a vast number of compounds with carbon, (the hydrocarbons), and an even larger array with heteroatoms that, because of their general association with living things, are called organic compounds. The study of their properties is covered in organic chemistry and their study in the context of living organisms is covered in biochemistry. By some definitions, "organic" compounds are only required to contain carbon. However, most of them also contain hydrogen, and because it is the carbon-hydrogen bond which gives this class of compounds most of its particular chemical characteristics, carbon-hydrogen bonds are required in some definitions of the word "organic" in chemistry. Millions of hydrocarbons are known, and they are usually formed by complicated synthetic pathways, which seldom involve direct reaction with elementary hydrogen.
Most molecular hydrides are volatile and many have simple structures that can be predicted by the VSEPR model. There are a large number of B hydrides known (boranes) and although the simplest BH3 has been found in the gas phase it readily dimerises to give B2H6.
In inorganic chemistry, hydrides can serve as bridging ligands that link two metal centers in a coordination complex. This function is particularly common in group 13 elements, especially in boranes (boron hydrides) and aluminium complexes, as well as in clustered carboranes, (composed of boron, carbon and hydrogen atoms). The bonding of the bridging hydrogens in many of the boranes is explained in terms of 3 centre - 2 electron bonds.
Diborane is a colourless and highly unstable gas at room temperature with a repulsively sweet odour. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature.
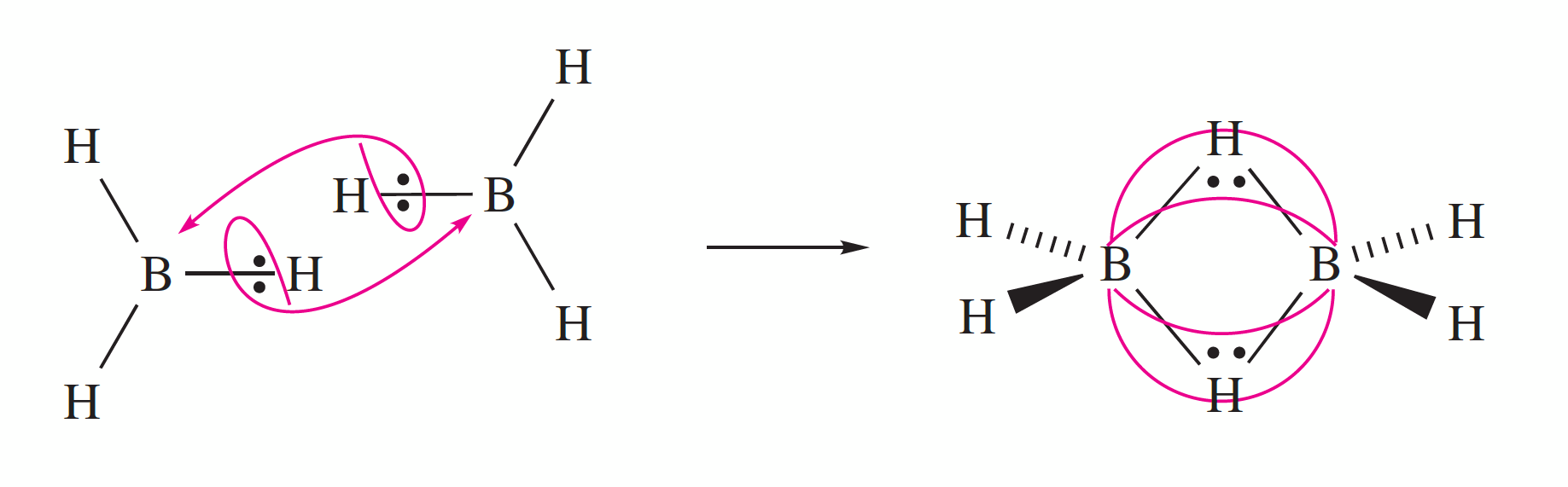 |
|
| MP = -164.85 °C, BP= -92.5 °C B-H (terminal) 119 pm, (bridge) 131 pm |
|
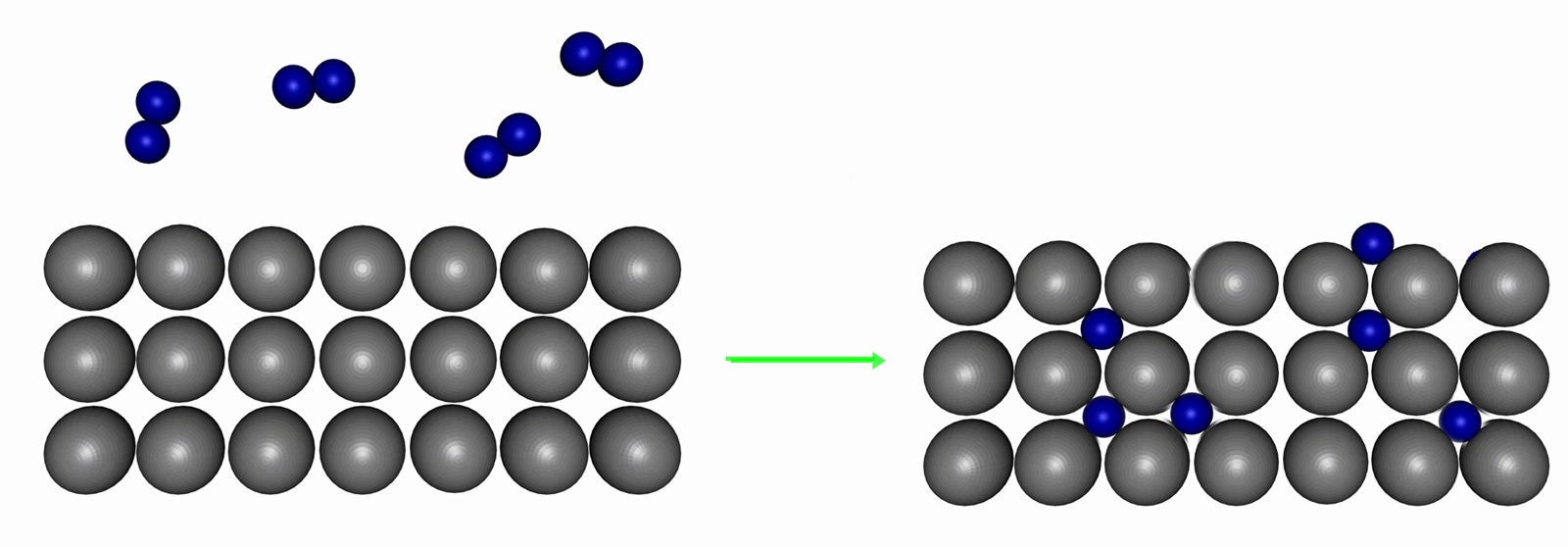
Metallic (interstitial) hydrides
Many transition metal elements form metallic (interstitial) hydrides, in which H2 molecules (and H atoms) can occupy the holes in the metal's crystal structure. They are traditionally termed 'compounds', even though they do not strictly conform to the definition of a compound; more closely resembling common alloys such as steel. These systems are usually non-stoichiometric, with variable amounts of hydrogen atoms in the lattice.
Palladium is unique in its ability to reversibly absorb large amounts of H2 or D2 (up to 900 times its own volume of hydrogen, but no other gases, at room temperature) to form palladium hydride. Structural studies show that the absorbed H fits into octahedral holes in the cubic close packed Pd lattice with a non-stoichiometric formula approximating to PdH0.6 for the β-form. This material has been considered as a means to carry hydrogen for vehicular fuel cells. Interstitial hydrides show some promise as a way for safe hydrogen storage. During the last 25 years many interstitial hydrides have been developed that readily absorb and discharge hydrogen at room temperature and atmospheric pressure. At this stage their application is still limited, as they are capable of storing only about 2 weight percent of hydrogen, insufficient for automotive applications.