10.6B: Trends in Boiling Points, Melting Points, and Enthalpies of Vaporization for p-block Binary Hydrides
- Page ID
- 34061
Hydrogen bonds
A hydrogen bond is the name given to the electrostatic attraction between polar molecules that occurs when a hydrogen (H) atom bound to a highly electronegative atom such as nitrogen (N), oxygen (O) or fluorine (F) experiences attraction to some other nearby highly electronegative atom. The name is something of a misnomer, as it represents a particularly strong dipole-dipole attraction, rather than a typical covalent bond.
The 2011 IUPAC definition specifies that "The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X-H in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation."
These hydrogen-bond attractions can occur between molecules (intermolecular) or within different parts of a single molecule (intramolecular). The hydrogen bond (5 to 30 kJ/mole) is stronger than a van der Waals interaction, but weaker than covalent or ionic bonds. This type of bond can occur in inorganic molecules such as water and in organic molecules like DNA and proteins.
Intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C) compared to the other group 16 hydrides that have no hydrogen bonds. Intramolecular hydrogen bonding is partly responsible for the secondary and tertiary structures of proteins and nucleic acids. It plays an important role in the structure of polymers, both synthetic and natural.
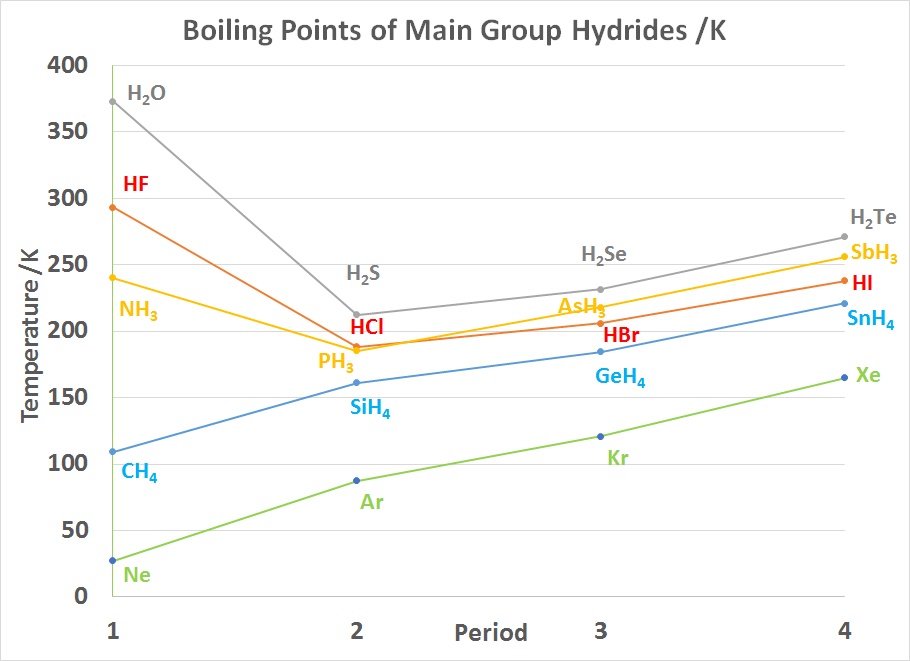
BP's of MG hydrides with Noble gases for comparison /K

