2.3b: MO theory of bonding in H₂⁺
- Page ID
- 2563
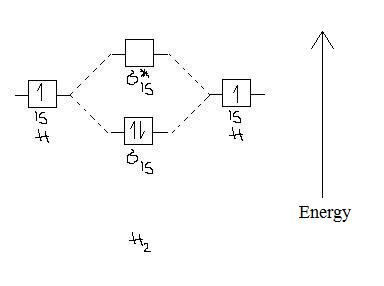
Note that there is a nodal plane in the anti-bonding MO.
Bond order
Bond order = 1/2 (#e- in bonding MO - #e- in antibonding MO)
For H2, bond order = 1/2 (2-0) = 1, which means H2has only one bond. The antibonding orbital is empty. Thus, H2 is a stable molecule.
Again, in the MO, there is no unpaired electron, so H2 is diamagnetic.
References
- Chang, Raymond. Physical Chemistry for the Biosciences. Sausalito, CA: University Science Books, 2005. 458 - 460.
- Housecroft, Catherine E. and Alan G. Sharpe. Inorganic Chemistry. 3rd ed. England: Pearson - Prentice Hall, 2008. 33 - 36.
Problems
- What does the MO of H2+ look like? What is its bond order? What is its magnetic property? Explain.
- What does the MO of H2- look like? What is its bond order? What is its magnetic property? Explain.
- Which one is the most stable: H2, H2+, or H2-? Why?
- When a hydrogen atom accepts an electron, it becomes a hydride H-. Theoretically would it be possible to form a molecule from two hydrides, that is to form H22-? Why?
Answers:
1-
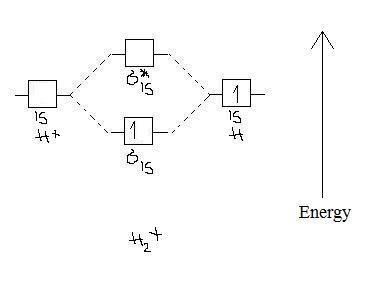
Bond order = 1/2 (1-0) = 1/2
Paramagnetic because it has one unpaired e- in the σ(1s) orbital.
2-
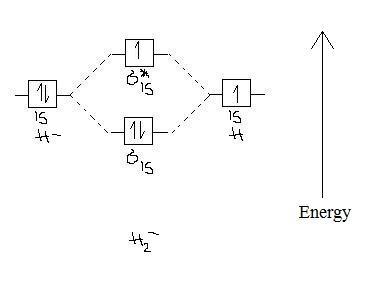
Bond order = 1/2 (2-1) = 1/2
Paramagnetic because it has one unpaired e- in the σ*(1s) orbital.
3- H2 is the most stable because it has the highest bond order (1), in comparison with the bond orders (1/2) of H2+ and H2-.
4- Theoretically it would not be possible to form a molecule from two hydrides because the anti-bonding and bonding orbitals would cancel each other out. So, the bond order is zero. Because the antibonding ortibal is filled, it destabilizes the structure, making the "molecule" H22- very non-stable.
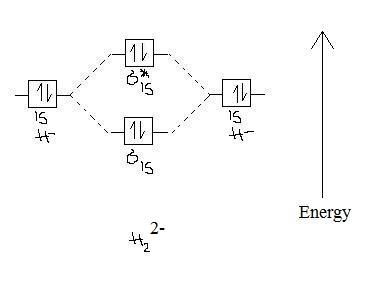
Bond order = 1/2 (2-2) = 0 ---> no bond formation. Thus, this molecule doesn't exist.

