5.2D: sp3 Hybridization
- Page ID
- 2580
Introduction
The term “sp3 hybridization” refers to the mixing character of one 2s-orbital and three 2p-orbitals to create four hybrid orbitals with similar characteristics. In order for an atom to be sp3 hybridized, it must have an s orbital and three p orbitals.
From wave function to the visual representation:
Four equivalent sp3 hybrid orbitals, resulting from the combination of one s atomic orbital and three p atomic orbitals, can then describe by four new wave functions (equations 1 – 4)
ψ(sp3) = 0.5 ( ψ2s + ψ2px + ψ2py + ψ2pz) (1)
ψ(sp3) = 0.5 ( ψ2s + ψ2px - ψ2py - ψ2pz) (2)
ψ(sp3) = 0.5 ( ψ2s - ψ2px - ψ2py + ψ2pz) (3)
ψ(sp3) = 0.5 ( ψ2s - ψ2px + ψ2py - ψ2pz) (4)
Plotting any of these four wave functions gives a picture representation of a sp3 orbital. Each hybrid orbital consists of a large lobe and a small lobe, pointing in two opposite direction (figure 1).
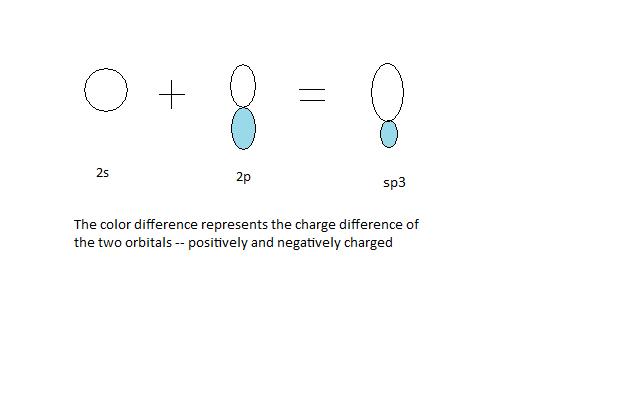
The Bond Angle is 109.5o:
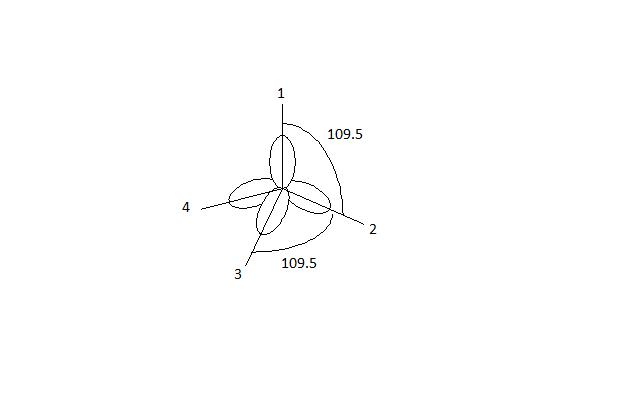
When the graphs of the four wave functions are combined, the resulting picture shows the tetrahedral arrangement of the four sp3 hybrid orbitals around the central atom. Because of the tetrahedral molecular geometry, the calculate bond angles between 1 and 2, 1 and 3, 1 and 4, 2 and 3, 2 and 4, and 3 and 4 approximately equal 109.5o (figure 2).
The Energy level and election population:
All four sp3 hybrid orbitals are delocalized—they occupy the same energy level; however, they are higher in energy than the 2s orbital and lower in energy than the 2p orbital (figure 3).
Just like any other atomic orbital, each sp3 hybrid orbital can house 2 elections.

S-character and the stability of the anion:
Each sp3 orbital has 1 part of s-character to 3 parts of p-character. In other words, it has 25% s-character and 75% p-character. Since the s orbital is closer to the nucleus and thus lower in energy than the p orbital, the electrons of sp3 hybridized species are held farther from the nucleus than those in sp2 (33% s-character) and sp (50% s-character) hybridized species. The closer the electrons are to the nucleus, the more stable they are. Therefore, when bearing the negative charge, sp3 species are less stable than sp2 and sp species. Put differently, sp3 species are less likely to get deprotonated (leaving a pair of electron behind).
Hybridization and bond length/bond strength:
The greater the s-character, the closer the electrons are held to the nucleus, the shorter the bond, and the stronger the bond. Thus, sp3 hybridized atoms form longer and weaker bonds than those of sp2 and sp hybridized.
References
- Brown W H, Foote C S, Iverson B L, Anslyn E V. Organic Chemistry, 5th Ed. Brooks/Cole Cengage Learning 2009, 2005.
Problems
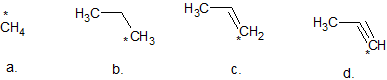
1. Which of the (*) carbons is/are sp3 hybridized
2. Draw the energy diagram for the orbitals of sp3 hybridzied carbon and nitrogen. Then fill in the correct number of electron.
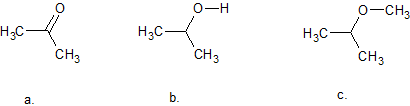
3. Indicate the hybridization of oxygen in each molecule
4. Which nitrogen atom(s) is/are sp3 hybridized
5. Describe the bonding scheme of CH4.
Answers:
1. a and b
2. Just like the energy diagram in fig.3.
For carbon, each sp3 orbital has 1 electron. For nitrogen, the first sp3 orbital has 2 electrons, then one electron for each of the remaining three
3. All of them (Don't for get the elctron pairs)
4. a and d
5. Carbon has four half-filled sp3 hybrid orbitals. Each orbital overlaps with a partially filled 1s atomic orbital of hydrogen to form 4 sigma bonds. To visualize, hydrogen atoms are placed at the four corner of the tetrahedron.
Contributors and Attributions
Quynh Nhu Nguyen

