5.1: History
- Page ID
- 275343
Coordination Chemistry
Now let us direct our focus toward actual coordination chemistry, also often called complex chemistry. In the following, we will apply the previously learned concepts about atomic theory, symmetry, molecular orbital theory, and acid-base chemistry to coordination compounds. Let us first ask: How are coordination compounds, or complexes defined? They are typically Lewis-acid base adducts between a metal atom or a metal ion as the Lewis acid and one or more ligands as a Lewis base. These ligands can be inorganic ligands such as halogenide ions, water, and ammonia molecules, or organic ligands like amines or alcohols. Coordination compounds are known since the antique due to their often intense color, and are used as a pigment or dye, for example Prussian Blue (KFe[Fe(CN)6] or tetrammine copper, both of which are intensely blue. You can see a few examples of coordination compounds below, namely the hexahydrate of copper (II) sulfate which is blue, iron (III) chloride, which is yellow, and nickel sulfate which is greenish-blue. Because of their intense color they have also attracted the attention of modern chemists from the early on, but the chemical bonding in these compounds remained a mystery for a relatively long time. The bonding in these compounds seemed more complex, hence the name complex compounds.

History on Coordination Compounds
What was the problem with the bonding in coordination compounds? Their empirical formulas could be easily determined by element analysis, but the results could not be explained with the concept of valence. In the early days of modern chemistry it was believed that the number of bonds in a compound could not exceed the valence. For example, in the compound of the formula Co(NH3)6Cl3 the valence of Co would be +3, therefore cobalt could not make more than three bonds. Assuming that the Co3+ ion would make three bonds to the 3 Cl- ions, how would one involve the six NH3 molecules in the bonding?

The chemist who solved this mystery was Alfred Werner. He correctly suggested that the number of bonds would not be restricted to the valence, but that more bonds would be allowed. It would be possible that the six NH3 molecules bind directly to Co3+ forming a so-called complex cation. They would be in the so-called first coordination sphere around the Co. The three chloride ions would then be bound loosely to the complex cation balancing the charge of the complex cation. They would be in the second coordination sphere.
Formula Writing in Coordination Chemistry
How can we write a formula of a coordination compound that contains complex ions?
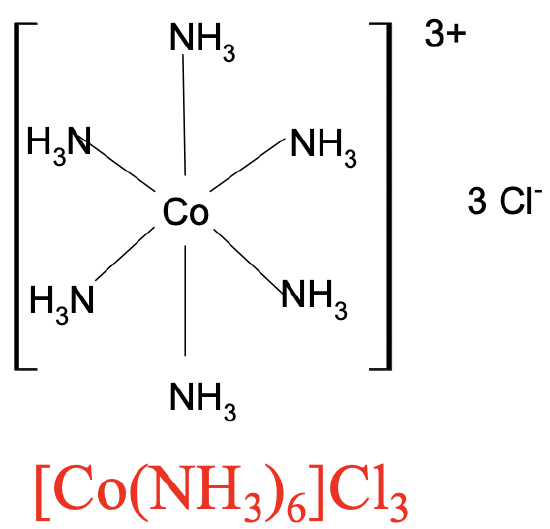
For complex cations we write the element symbol for the metal ion first followed by the formula for the ligands. If there is more than one ligand, then we will place the ligands in parentheses, and indicate their number by a subscript behind the parentheses. The entire complex cation is placed in brackets. The formula for the anion in the second coordination sphere is placed behind the brackets and the number of anions is indicated by a subscript (Fig. 5.1.3).

For complex anions, we write the formula for the counter cation first, followed by the complex anion in brackets. You can see two examples above (Fig. 5.1.3 and 5.1.4). We have complex cations of Co with six NH3 ligands coordinated to it in the first coordination sphere. Three Cl- ions are in the second coordination sphere. Hence the formula is [Co(NH3)6]Cl3. The second example is the coordination compound with the complex anion having a 3- charge in which six cyanide anions bind to a central Fe3+ ion. The K+ ion in the second coordination sphere compensates the charge of the complex anion. Hence the formula is K3[Fe(CN)6].
Dr. Kai Landskron (Lehigh University). If you like this textbook, please consider to make a donation to support the author's research at Lehigh University: Click Here to Donate.


