5.1: Group 1 Metals
- Page ID
- 125399
Group 1 metals are called alkali metals. Alkali metals are abundant in minerals and sea water. Especially the content of sodium, Na, in the Earth's crust is fourth after Al, Fe, and Ca. Although the existence of sodium or potassium ions was recognized for many years, a number of attempts to isolate the metals from aqueous solutions of their salts failed because of their high reactivity with water. Potassium (1807) and subsequently sodium were isolated by the electrolysis of molten salt of KOH or NaOH by H. Davy in the 19th century. Lithium Li was discovered as a new element (1817), and Davy soon isolated it by molten salt electrolysis of Li2O. Rubidium, Rb and Cesium, Cs, were discovered as new elements by spectroscopy in 1861. Francium, Fr, was discovered using a radiochemical technique in 1939. Its natural abundance is very low.
| mp (°C) |
bp (°C) |
d(20 °C) (g cm-3) |
E0 (V) M+ + e- |
I (kJ mol-1) |
|
|---|---|---|---|---|---|
| Li | 181 | 1342 | 0.534 | -3.04 | 520 |
| Na | 98 | 883 | 0.968 | -2.71 | 496 |
| K | 63 | 759 | 0.856 | -2.93 | 419 |
| Rb | 39 | 688 | 1.532 | -2.98 | 403 |
| Cs | 28 | 671 | 1.90 | -3.03 | 376 |
As shown in Table \(\PageIndex{1}\), melting-points, boiling points, and densities of alkali metals are low, and they are soft metals. Since the outer shell contains only one s-electron, the ionization energy is very low, and mono cations of alkali metals form easily. Qualitative analysis of alkali metals is possible by means of flame reactions using characteristic luminescence lines. Especially the orange D-line of sodium is used in the sodium lamp. Alkali metals are oxidized by water evolving hydrogen gas due to their low reduction potentials. Except lithium, the heavier alkali metals react violently with water, and sufficient caution should be exercised in their handling.
Exercise \(\PageIndex{1}\)
Describe the reactivity of alkali metals in water.
- Answer
-
The reactivity of lithium is the lowest, sodium reacts violently, and potassium, rubidium, and cesium react explosively.
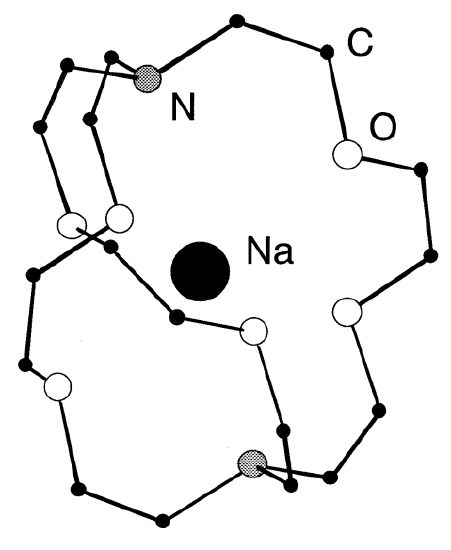
Alkali metals are also highly reactive to oxygen or halogens. As alkali metals are very reducing, they are used widely as reducing agents. Because of the high affinity of alkali metals to halogens, they are important in organic and inorganic syntheses which produce alkali metal halides as the result of condensation and metathesis reactions. Although it is generally difficult to dissolve metals in solvents to make atomic dipersions, alkali metals can be dispersed in liquid ammonia solutions, amalgams, and as cryptand (Figure \(\PageIndex{1}\)), naphthalene, or benzophenone (C6H5)2CO complexes.
Ammonia boils at -33.35 °C but liquid ammonia can be easily handled. Alkali metals dissolve readily in liquid ammonia and dilute solutions are blue but concentrated ones show a bronze color. The metal is recovered when ammonia is evaporated from metal solutions. Alkali metal solutions show the same color irrespective of the kind of alkali metals as the color is due to the solvated electrons. Namely, the dissolution is accompanied by the separation of the alkali metal atoms into metal cations and electrons solvated by ammonia, according to the following equation.
\[M + n\; NH_{3} \rightarrow M^{+} [e^{-} (NH_{3})]\]
The liquid ammonia solution of an alkali metal is conductive and paramagnetic. The highly reducing solution is used for special reduction reactions or syntheses of alkali metal complexes and polyhalides.

