5.1: Organometallic Compounds of Zinc and Cadmium
- Page ID
- 177781
Learning Objectives
In this section you will learn the following
- Organometallic compounds of zinc and cadmium.
- Structural features of organozinc compounds
Organometallic compounds of zinc and cadmium
Dialkyl compounds of Zn, Cd and Hg do not associate through alkyl bridges.
Dialkylzinc compounds are only weak Lewis acids, organocadmium compounds are even weaker, and organomercury compounds do not act as Lewis acids except under special circumstances.
The Group 12 metals form linear molecular compounds, such as ZnMe2, CdMe2 and HgMe2, that are not associated in solid, liquid or gaseous state or in hydrocarbon solution.
They form 2c, 2e bonds. Unlike Be and Mg analogs, they do not complete their valence shells by association through alkyl bridges. The bonding in these molecules are similar to d10 metals such as CuI, AgI and AuI with linear geometry ([N≡C-M-C≡N]-, M = Ag or Au). This tendency is sometimes rationalized by invoking pd hybridization in the M+ ion, which leads to orbitals that favor linear attachment of ligands (similar to spd hybridization).
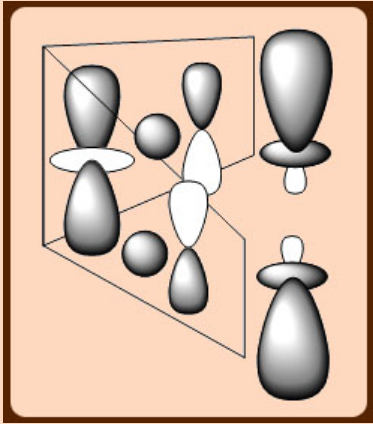
The preference for the linear coordination may be due to the similarity in energy of the outer ns, np and (n-1)d orbitals, which permits the formation of collinear spd hybrids.
The hybridization of s, pz and dz2 with the choice of phases shown here produces a pair of collinear orbitals that can be used to form strong σ-bonds.
Organozinc and organocadmium compounds
Convenient route is metathesis with alkylaluminium or alkyllithium compounds.
With alkyllithium compounds it is the electronegativity which is decisive, whereas between Al and Zn it is hardness considerations correctly predict the formation of softer ZnCH3 and harder AlCl pairs.
\[\ce{ZnCl2 + Al2Me6 -> ZnMe2 + Al2Cl2Me4} \nonumber \]
Alkylzinc compounds are pyrophoric and readily hydrolyzed, whereas alkylcadmium compounds react more slowly with air. Due to mild Lewis acidity, dialkylzinc and dialkylcadimum compounds form stable complexes with amines, especially with chelating amines.
The Zn—C has greater carbanionic character than the Cd—C bond.
For example, addition of alkylzinc compounds across the carbonyl group of a ketone:
\[\ce{ZnMe2 + (CH3)2C=O -> (CH3)2C-O-ZnCH3} \nonumber \]
This reaction do not proceed with the less polar alkylcadmium or alkylmercury compounds, but organolithium, organomagnesium and organoaluminium compounds can promote this reaction readily since all of which contain metals with lower electronegativity than zinc.
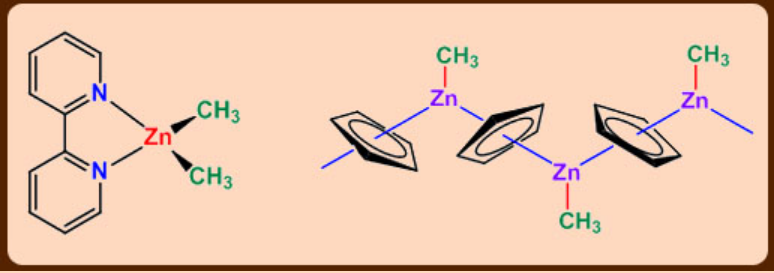
Interestingly, the cyclodipentadienyl compounds are structurally unusual. CpZnMe is monomeric in the gas phase with a pentahapto Cp group.
In the solid state it is associated in a zig-zag chain, each Cp group being pentahapto with respect to two Zn atoms.
Problems:
1. Do you think that the following reaction proceeds? If so, why and how?
\[\ce{ZnCl2 + Al2Me6 -> ZnMe2 + Al2Cl2Me4} \nonumber \]
Solution
Al2Me6 being an electron deficient molecule readily exchanges two methyl groups with zinc for two chloride ions. Since chloride ions have sufficient electron in their valence shell act as four electron donor through bridging coordination mode. Al2Cl2Me4 is no longer an electron deficient molecule.


