4.2: Organometallic Compounds of As(III) and Sb(III)
- Page ID
- 172739
Learning Objectives
In this lecture you will learn the following
- Preparation of trivalent compounds.
- Mono and bis derivatives.
- Reaction of organo arsenic and antimony compounds.
- Structural features of organolead compounds.
Organometallic compounds of As(III) and Sb(III)
Direct synthesis
Mono- derivatives
\[\ce{2As + 3MeBr ->[\Delta T][Cu] Me2AsBr + MeAsBr2} \nonumber \]
\[\ce{Me2AsBr + PhLi -> Me2AsPh} \nonumber \]
\[\ce{MeAsBr2 + 2PhLi -> MeAsPh2} \nonumber \]
\[\ce{EX3 + 3RMgX -> R3E + 3MgX2} \nonumber \]
\[\ce{EX3 + 3RLi -> R3E + 3LiX} \nonumber \]
Bis derivatives:
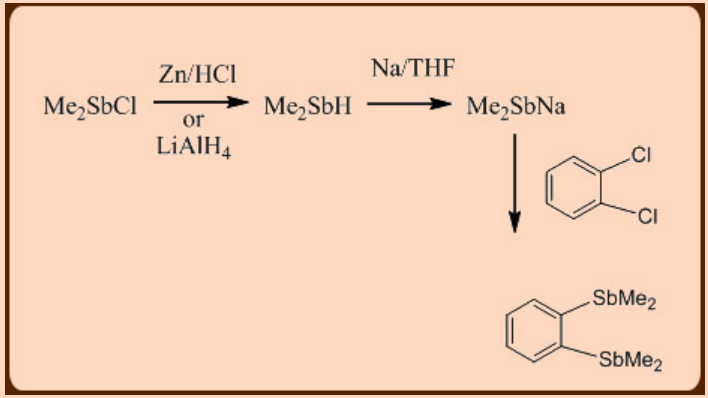
In a similar way, a variety of bisphosphines and arsines can be generated.
Reactions of trialkyl derivatives, R3E
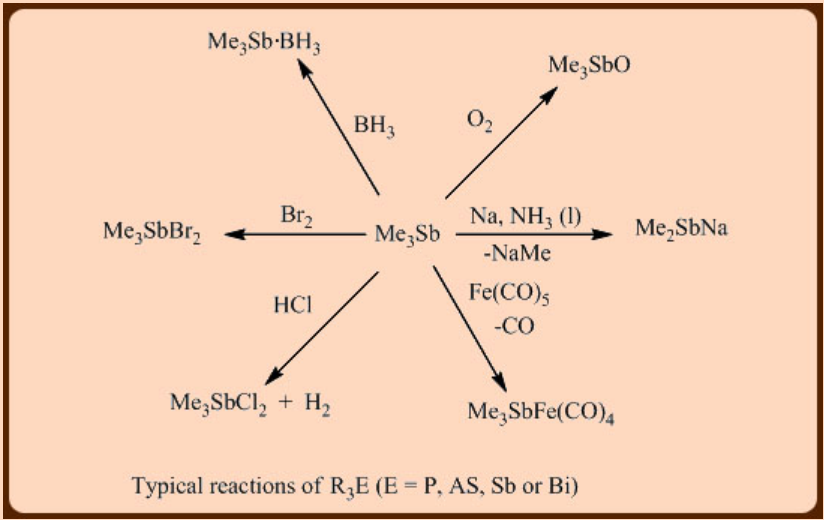
The transition metal chemistry of R3E, phosphines, arsines or stibines has been extensively studied because of their distinct donor and acceptor properties. Among them, the phosphines or tertiary phosphines (R3P) are the most valuable ligands in metal mediated homogeneous catalysis. Interestingly, the steric and electronic properties can be readily tuned by changing the substituents on phosphorus atoms. Chapter 16 is fully dedicated to the chemistry of phosphines.
Properties
Trialkyl derivatives are highly air-sensitive liquids with low boiling points and some of them are even pyrophyric. Triphenyl derivatives are solids at room temperature and are moderately stable and oxidizing agents such as KMnO4, H2O2 or TMNO are needed for oxidation to form Ph3E=O.
Cyclic and acyclic derivatives containing E—E bonds
E—E single bonds:
The E—E bond energies suggest that they do not have greater stability and the stability decreases down the group.
The simplest molecules include Ph2P—PPh2, Me2As—AsMe2 prepared by coupling reactions:
\[\ce{Me2AsH + Me2AsCl ->[ ][-HCl] Me2As-AsMe2} \nonumber \]
\[\ce{2Ph2BiCl ->[Na, NH3] Ph2Bi-BiPh2} \nonumber \]
The weakness of E—E bonds accounts for many interesting reactions and a few of such reactions are listed below:
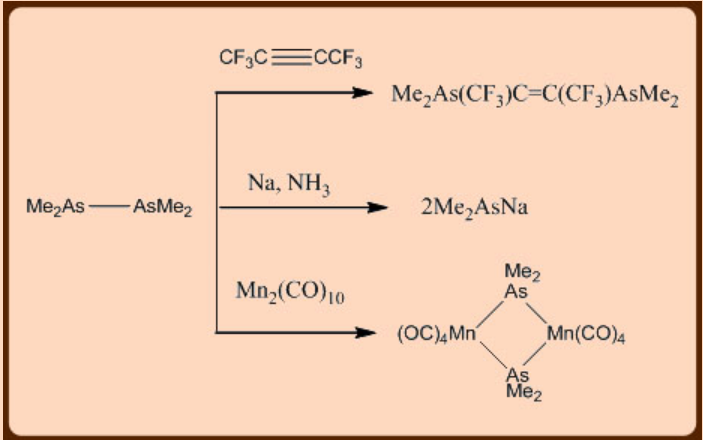
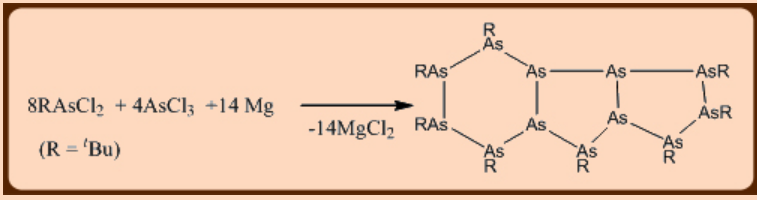
Cyclic and polycyclic derivatives can be prepared by employing any of the following methods:
Problems:
1. Confirm that the octahedral structure of [Ph6Bi]- is consistent with VSEPR theory.
Solution:
Octahedral similar to PF6-
5 (Bi valence electrons) + 6 (each Ph ) + 1 (-ve charge) = 12 electrons
i.e. six pairs, octahedral geometry
2. Comment on the stability of BiMe3 and Al2(iBu)6 with respect to their thermal decomposition and give chemical equations for their decomposition.
Solution:
Similar to other heavy p-block elements, Bi—C bonds are weak and readily undergo homolytic cleavage. The resulting methyl radicals will react with other radicals or form ethane.
\[\ce{2BiMe3 -> 2Bi + 3CH3-CH3} \nonumber \]
The Al2(iBu)6 dimer readily dissociates. At elevated temperature dissociation is followed by β-hydrogen elimination. This type of elimination is common for organometallic compounds that have alkyl groups with β-hydrogens, can form stable M—H bonds, and can provide a coordination site on the central metal.
The decomposition reaction is:
\[\ce{Al2(iBu)6 ->[\Delta] 2Al(iBu)3 -> [\Delta \Delta] Al(iBu)2H + (CH3)2C=CH2} \nonumber \]
- Using a suitable Grignard reagent, how would you prepare (i) MeC(Et)(OH)Ph; (ii) AsPh3.
Solution
- Add a Grignard reagent to a C=O bond, then acidify.
Several possibilities, e.g. \[\ce{Me-C(O)-Et + PhMgBr -> Me-C(OMgBr)(Et)(Ph) -> MeC(Et)(OH)Ph \: or \: Me-C(O)-Ph + EtMgBr -> etc} \nonumber \] - \[\ce{AsCl3 + 3PhMgBr -> AsPh3 + 3MgBrCl} \nonumber \]
- Add a Grignard reagent to a C=O bond, then acidify.


