3.6: Organotin and Organolead Compounds
- Page ID
- 172736
Learning Objectives
In this section you will learn the following
- Organotin and organolead compounds and their preparation.
- Bonding in tin compounds with Sn=Sn double bonds.
- Uses and environmental issues with tin compounds.
- Reactivity of tetraethyl lead.
- Structural features of organolead compounds.
Organotin and organolead compounds
Preparation of Sn(IV) derivatives
\[\ce{3SnCl3 + 4R3Al ->[R'2O] 3R4Sn + 4AlCl3} \nonumber \]
\[\ce{R4Sn + SnCl4 -> R3SnCl + RSnCl3 ->[500K] 2R2SnCl2} \nonumber \]
\[\ce{SnCl4 + 4RMgBr -> R4Sn + 4MgBrCl} \nonumber \]
\[\ce{SnCl2 + Ph2Hg -> Ph2SnCl2 + Hg} \nonumber \]
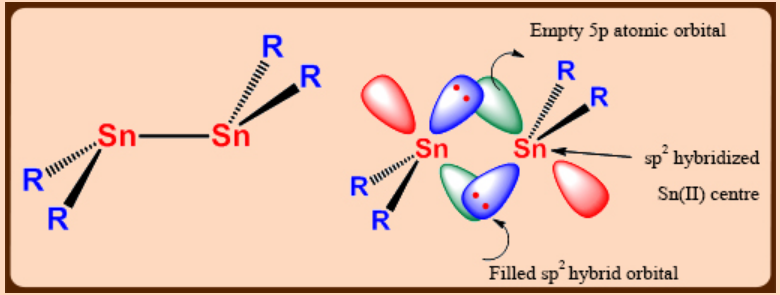
Tin(II) organometallics of the type R2Sn, containing Sn-C σ-bonds, are stabilized only if R is sterically demanding.
\[\ce{SnCl2 + 2Li[(Me3Si)2CH] -> [(Me3Si)2CH]2Sn} \nonumber \]
(monomeric in solution and dimeric in solid state). But the dimer does not possess a planar Sn2R4framework unlike an analogous alkene, and Sn—Sn bond distance (267 pm) is shorter than a normal Sn—Sn single bond (276 pm).
Sn2R4 has a trans bent structure with a weak Sn=Sn double bond
Look into the reactions of R3SnCl with various reagents to form useful tin containing starting materials
The first organotin(II) hydride was reported only in 2000.
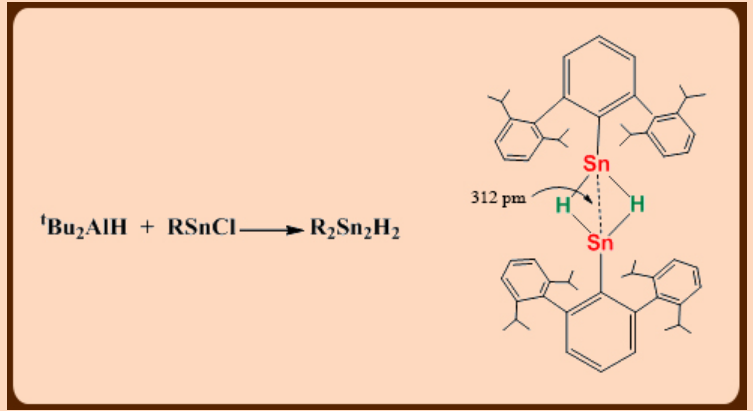
Shows dimeric structure in the solid state containing hydride bridges (Sn-Sn = 312 pm).
Commercial uses and environmental problems
Organotin(II) compounds find wide range of applications due to their catalytic and biocidal properties.
- nBu3SnOAc is an effective fungicide and bactericide and also a polymerization catalyst.
- nBu2Sn(OAc)2 is used as a polymerization catalyst and a stabilizer for PVC.
- nBu3SnOSnnBu3 is algicide, fungicide and wood-preserving agent.
- nBu3SnCl is a bactericide and fungicide.
- Ph3SnOH used as an agricultural fungicide for crops such as potato, sugar beet and peanuts.
- The cylic compound (nBu2SnS)3 is used as a stabilizer for PVC.
Tributyltin derivatives have been used as antifouling agents, applied to the underside of ships’ hulls to prevent the build-up of, for example, barnacles.
Global legislation now bans or greatly restricts the use of organotin-based anti-fouling agents on environmental grounds. Environmental risks associated with the uses of organotin compounds as pesticides, fungicides and PVC stabilizers are also a cause for concern.
*A barnacle is a type of arthropod belonging to infraclass Cirripedia in the sub-phylum Crustacea, and is hence related to crabs and lobsters.
Organolead compounds
Tetraethyllead
\[\ce{4NaPb + 4EtCl -> Et4Pb + 3Pb + 4NaCl} [at\: 373K\: in\: an\: autoclave] \nonumber \]
Laboratory Scale,
\[\ce{2PbCl3 + 4RMgBr ->[Et2O][-4MgBrCl] 2(R2Pb) -> R4Pb + Pb} \nonumber \]
Thermolysis leads to radical reactions.
\[\ce{Et4Pb -> Et3Pb + Et} \nonumber \]
\[\ce{2Et -> n-C4H10} \nonumber \]
\[\ce{Et3Pb + Et -> C2H4 + Et3PbH} \nonumber \]
\[\ce{Et3Pb + Et4Pb -> H2 + Et3Pb + Et3PbCH2CH2} \nonumber \]
Tetraalkyl and tetraaryl lead compounds are inert with respect to attack by air and water at room temperature. WHY ????
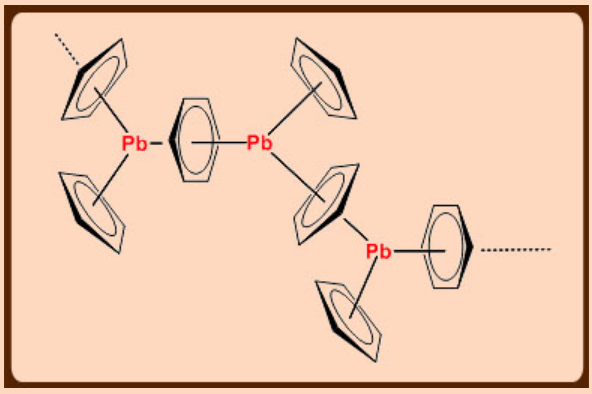

Me3PbCl consists of linear chain
Solid state structure of Cp2Pb shows polymeric nature, but in the gas phase, discrete Cp2Pb molecules are present which possess the bent structure similar to silicon analogue.
R2Pb=PbR2 are similar to analogues tin compounds
Problems
- Find out the structures of (Me3SiCH2)3SnF and Me2SnF2
Solution: use VSEPR theory


