3.5: Organosilicon and Organogermanium Compounds
- Page ID
- 172735
Learning Objectives
In this lecture you will learn the following
- Organosilicon and organogermanium compounds.
- Compounds with Si=Si and Ge=Ge bonds.
Organosilicon and organogermanium compounds
Organosilicon compounds are extensively studied due to the wide range of commercial applications as water repellents, lubricants, and sealants. Many oxo-bridged organosilicon compounds can be synthesized. e.g. (CH3)3Si—O—Si(CH3)3 which is resistant to moisture and air.
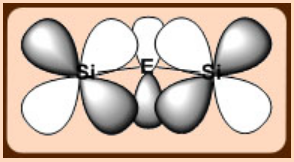
The lone pairs on O are partially delocalized into vacant σ*- orbitals of Si, as a result the directionality of the Si-O bond is reduced making the structure more flexible.
This flexibility permits silicone elastomers to remain rubber-like down to very low temperature.
Delocalization also accounts for low basicity of an O atom attached to silicon as the electrons needed for the O atom to act as a base are partially removed.
The planarity of N(SiH3)3 is also explained by the delocalization of the lone pair on N which makes it very weakly basic.
\[\ce{nMeCl + Si/Cu -> Me_{n}SiCl_{4-n}} \nonumber \]
\[\ce{SiCl4 + 4RLi -> R4Si} \nonumber \]
\[\ce{SiCl4 + RLi -> RSiCl3} \nonumber \]
\[\ce{SiCl4 + 2RMgCl -> R2SiCl2 + 2MgCl2} \nonumber \]
\[\ce{Me2SiCl2 + tBuBi -> tBuMe2SiCl + LiCl}|]
Si—C bonds are relatively strong (bond enthalpy is 318 kJ mol-1) and R4Si derivatives possess high thermal stabilities. Et4Si on chlorination gives (ClCH2CH2)4Si, in contrast to the chlorination of R4Ge or R4Sn which yields RnGeCl4-n or RnSnCl4-n.
Me2SiCl2 on hydrolysis produce silicones.
\[\ce{Me3SiCl + NaCp -> (η^{1}-Cp)SiHMe3} \nonumber \]
(η1-C5Me5) 2SiBr2 on treatment with anthracene/potassium gives Cp*2Si
Solid state structure of Cp*2Si consists of two independent molecules which differ in the relative orientations of the Cp rings.
In one molecule, they are parallel and staggered whereas in the other, they are tilted with an angle of 167°at Si.
The reaction between R2SiCl2 and alkali metal or alkali naphthalides give cyclo-(R2Si)n by loss of Cl-and Si—Si bond formation.
Bulky R groups favour small rings [e.g. (2,6-Me2C6H3)6Si3 and tBu6Si3] while smaller R groups encourage the formation of large rings [Me12Si6, Me14Si7 and Me32Si16]
\[\ce[Ph2SiCl2 + Li(SiPh2)5Li -> cyclo-Ph12Si6 + 2LiCl} \nonumber \]
Bulky substituents stabilize R2Si=SiR2 compounds. The sterically demanding 2,4,6-iPr3C6H2 provided first example of compound containing conjugated Si=Si bonds.
Has s-cis configuration in both solution and the solid state.
Similar germanium compounds are also known
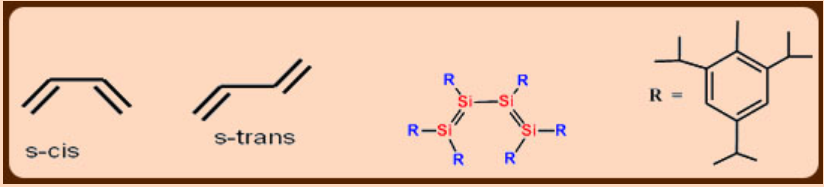
*The spatial arrangement of two conjugated double bonds about the intervening single bond is described as s- cis if synperiplanar and s-trans if antiperiplanar.


