3.4: Zeigler Natta Polymerization Catalysts
- Page ID
- 172734
Learning Objectives
In this lecture you will learn the following
- Olefin polymerization.
- Mechanism involved in polymerization process.
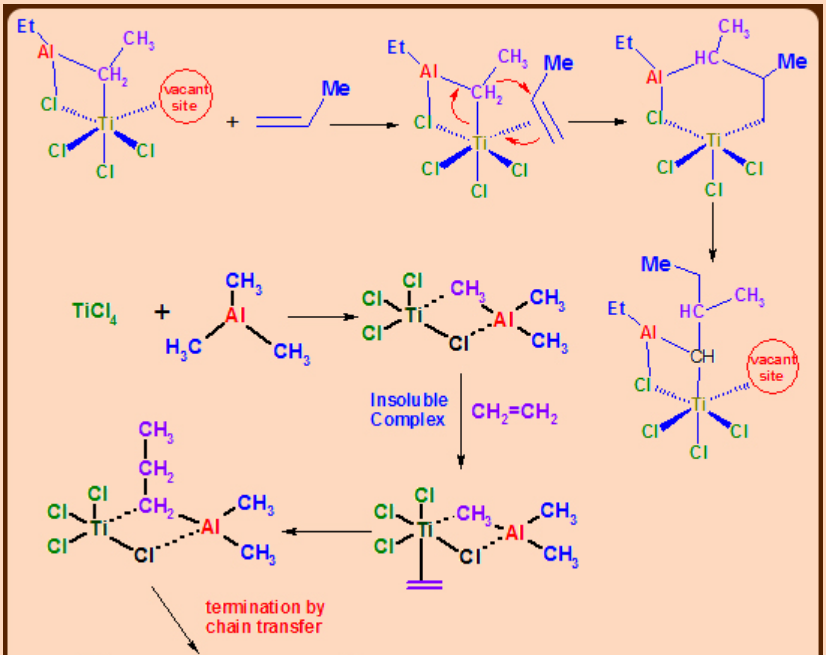
Ziegler Natta Polymerization Catalysts


Insertion of aluminum alkyls into olefins was studied by Ziegler. During the systematic investigation of olefin polymerization, Ziegler realized that the most effective catalyst is the combination of TiCl4/AlEt3which can polymerize ethylene at pressure as low as 1 bar. The application of Ziegler method to the polymerization of propylene and its establishment and the investigation of bulk properties was carried out by Natta and hence the methodology is called Ziegler-Natta process.
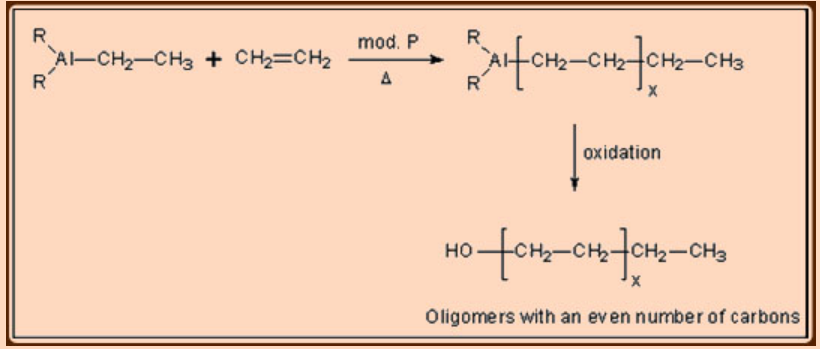
Important discovery: R3Al + Lewis acids.
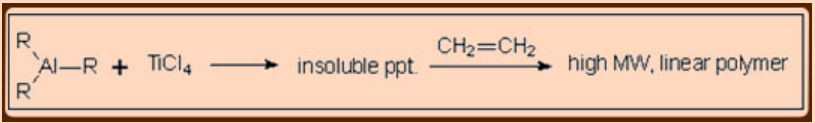
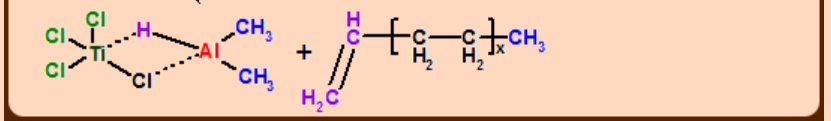
In the absence of reaction mechanism with solid proof, it is presumed that the reaction is due to the heterogeneous catalysis in which fibrous TiCl3, alkylated on its surface is considered to be the active catalyst species.