3.1: Reactions of Organometallic Compounds
- Page ID
- 172731
Learning Objectives
In this section you will learn the following
- Chemical properties of organometallic compounds.
- Reactivity patters and their electronic properties.
- Electronic properties verses chemical reactivity.
- Application of alkyl lithium in organic transformations.
Reactions of organometallic compounds
The reactions of organometallic compounds of electropositive elements are dominated by factors such as the carbanion character of the organic moiety and the availability of a coordination site on the central metal atom. Reaction patterns:
a. Oxidation
- All organometallic compounds are potentially reducing agents.
- Those of electropositive elements are in fact very strong reducing agents (many of them are pyrophoric in nature).
- The strong reducing character also presents a potential explosion hazard if the compounds are mixed with larger amount of oxidzing agents.
Why is it so?
All organometallic compounds of the electropositive metals that have unfilled valence orbitals, or that readily dissociate into fragments with unfilled orbitals are pyrophoric,e.g., \(\ce{Li4(CH3)4}\), \(\ce{Zn(CH3)2}\), \(\ce{B(CH3)3}\) and \(\ce{Al2(CH3)6}\)
Volatile pyrophoric compounds, such as \(\ce{B(CH3)3}\), may be handled in vacuum line and, inert atmosphere techniques are used for less volatile but air-sensitive compounds. Compounds such as \(\ce{Si(CH3)4}\) and \(\ce{Sn(CH3)4}\) which do not have low-lying empty orbitals, require elevated temperatures to initiate combustion, and can be handled in air.
The combustion of many organometallic compounds takes place by a radical chain mechanism.
b. Nucleophilic character
The partial negative charge of an organic group attached to an electropositive metal makes it a strong nucleophile and Lewis base. This is referred to as its carbanion character even though the compound itself is not ionic. Alkyllithium and alkylaluminium compounds and Grignard reagents are the most common carbanion reagents in laboratory-scale synthetic chemistry. The carbanion character diminishes for the less metallic boron and silicon.
The carbanion character finds many synthetic applications.
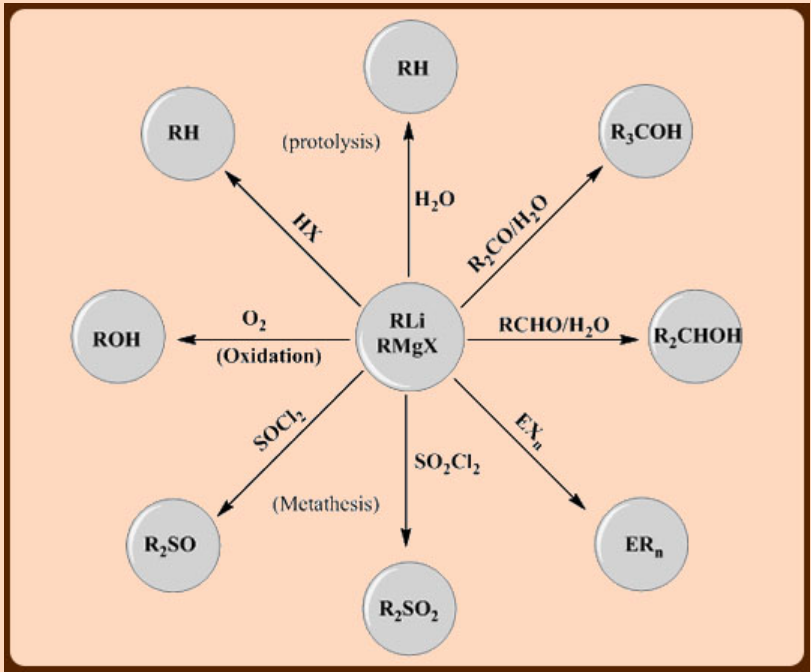
Where X = halide, E = B, Si, Ge, Sn, Pb, As and Sb
c. Lewis acidity
Due to the presence of unoccupied orbitals on the metal atom, electron-deficient organometallic compounds are observed to be Lewis acids.
e.g \[\ce{B(C6H5)3 + LiC6H5 -> Li[B(C6H5)4]} \nonumber \]
This reaction may be viewed as the transfer of the strong base C6H5-from the weak Lewis acid Li+to the stronger acid B(III).
Organometallic species that are bridged by organic groups can also serve as Lewis acids and, in the process, bridge cleavage can take place.
\[\ce{Al2(CH3)6 + 2N(C2H5)3 -> 2(CH3)3AlN(C2H5)3} \nonumber \]
Electron deficient organometallic species are Lewis acids.
Problems
1. Name each of the following compounds and classify them: (a) SiH(CH3)3, (b) BCl(C6F5)2, (c) Al2Cl2(C6H5)4, (d) Li4(C4H9)4, (e) Rb(CH2H3).
Solution
- trimethylsilane (tetrahedral monomer, electron-precise);
- bis(pentafluorophenyl)chloroborane (trigonal monomer, electron-deficient);
- tetraphenyldichlorodialuminum (two Al-Cl-Al bridges, in this structural form it is electron –precise);
- butyllithium or more precisely, tetrabutyltetralithium (tetrahedral Li4 array with a phenyl carbon bridging each face, electron deficient);
- ethylrubidium, salt-like.
2. Sketch the structures of: (a) methyl lithium, (b) trimethyl boron, (c) hexamethyldialuminum, (d) tetramethylsilane, (e) trimethylarsane, and (f) tetraphenylarsonium.
Solution
- methyl lithium: Li tetrahedron with each face capped by CH3 [see chapter 6]
- trimethyl boron: planar triangular array of B and C
- hexamethyldialuminum:four terminal CH3 and two CH3 bridges in a diborane-like structure
- tetramethylsilane: tetrahedral
- trimethylarsane: pyramidal
- tetraphenylarsonium: pseudotetrahedral


