2.3: Organometallic Compounds of Alkaline Earth Metals (Beryllium and Magnesium)
- Page ID
- 172726
Objectives
In this lecture you will learn the following
- Organometallic compounds of beryllium and magnesium.
- Structural features of alkyl lithium and beryllium sandwich compounds.
Beryllium
\[\ce{HgMe2 + Be -> Me2Be + Hg (at \: 383K)} \nonumber \]
\[\ce{2PhLi + BeCl2 -> Ph2Be +2LiCl (in\:diethyl\:ether)} \nonumber \]
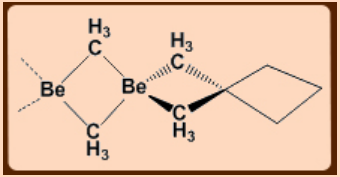
In vapor phase, Me2Be is monomeric with a linear C—Be—C (Be-C = 170 pm).
The solid state structure is polymeric and resembles that of BeCl2.
\[\ce{2NaCp + BeCl2 -> Cp2Be + 2NaCl} \nonumber \]
The X-ray diffraction at 128 K suggested [(η1-Cp)(η5-Cp)Be].
However, 1H NMR spectrum shows that all protons environments are equivalent even at 163 K.
Also, solid state structure shows the Be atom is disordered over two equivalent sites and NMR data can be interpreted in terms of fluxional process in which the Be atom moves between these two sites.
However, Cp*2Be possesses a sandwich structure with both the rings are coplanar.
Magnesium
Alkyl and aryl magnesium halides (Grignard reagents, RMgX) are extremely well-known on account of their uses in synthetic chemistry.
\[\ce{Mg + Rx -> RMgX (in\:diethyl\:ether)} \nonumber \]
Transmetallation is useful means of preparing pure Grignard reagents.
\[\ce{Mg + RHgBr -> Hg + RMgBr} \nonumber \]
\[\ce{Mg + R2Hg -> Hg + R2Mg} \nonumber \]
Two-coordination at Mg in R2Mg is observed only when the R groups are sufficiently bulky, e.g. Mg{C(SiMe3)3}2.
RMgX are generally solvated and Mg centre is typically tetrahedral.
e.g. EtMgBr.2Et2O; PhMgBr.2Et2O.
Cp2Mg has a staggered sandwich structure.
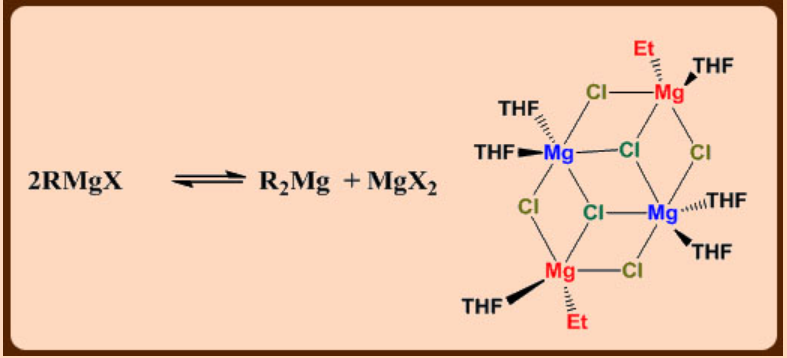
Solutions of Grignard reagent may contain several species, e.g. RMgX, R2Mg, MgX2, RMg(μ-X)2MgR, which are further complicated by solvation. The position of equilibrium between these species is markedly dependent on concentration, temperature and solvent; strongly donating solvents favour monomeric species in which they coordinate to the metal centre.
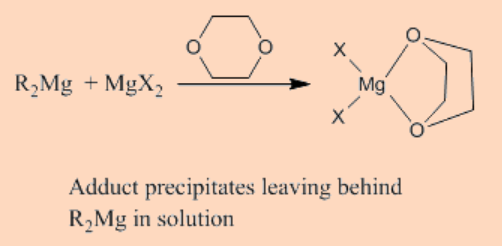
Treatment with dioxane results in the precipitation of MgCl2(dioxane) leaving behind pure R2Mg in the solution.
Problems:
1. The compound (Me3Si)2C(MgBr)2.nTHF is monomeric. Suggest a value of ‘n’ and propose a structure for this Grignard reagent.
Solution
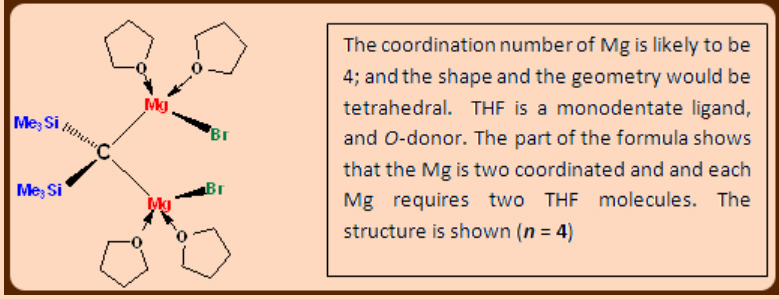
2. If a typical Grignard reagent exists as an equilibrium mixture of dialkylmagnesium and magnesium halide, give a method of isolating pure dialkyl magnesium. Your answer should be in the form of balanced chemical equations only.
Solution
\[\ce{2RMgX <=> R2Mg + MgX2} \nonumber \]
Treatment of equilibrium mixture with dioxane results in the precipitation of, say, MgCl2(dioxane) (if, X = Cl), leaving behind pure R2Mg in the solution.