2.2: Organometallic Compounds of Alkali Metals (Sodium and Lithium)
- Page ID
- 172725
Learning Objectives
In this lecture you will learn the following
- How to synthesize and handle sodium, lithium compounds.
- Structural features.
Organometallic compounds of alkaline metals
Organic compounds such as terminal alkynes which contain relatively acidic hydrogen atoms form salts with the alkali metals.
\[\ce{2Et-C≡CH + 2Na -> 2Na+[Et-C≡C]- + H2} \nonumber \]
\[\ce{Me-C≡C-H + K[NH2] -> K+[Me-C≡C]- + NH3} \nonumber \]
\[\ce{C5H6 + Na -> NaCp + 1/2 H2} \nonumber \]
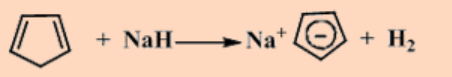
NaCp is pyrophoric in air, but air-sensitivity can be lessened by complexing the Na+ with dme.
In the solid state, [Na(dme)][Cp] is polymeric
*Pyrophoric material: is one that burns spontaneously when exposed to air.
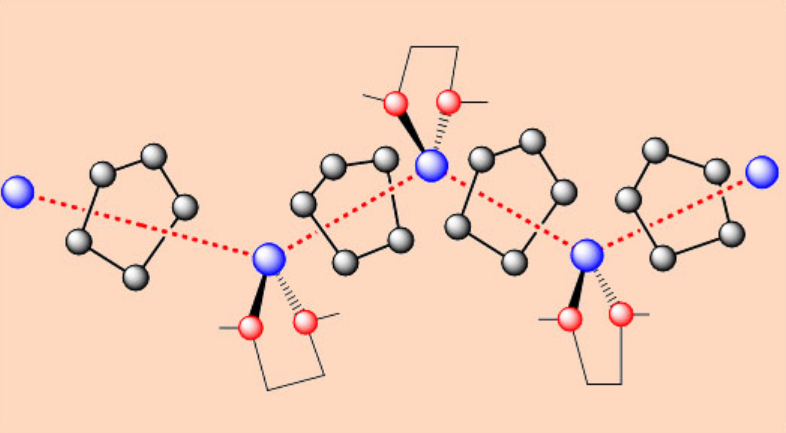
Transmetallation:
\[\ce{HgMe2 + Na -> 2NaMe + Hg} \nonumber \]
Organolithium compounds:
\[\ce{nBuCl + 2Li ->[Hydrocarbon\:Solvent] nBuLi + LiCl} \nonumber \]
Organolithium compounds are of particular importance among the group 1 organometallics.
Many of them are commercially available as solutions in hydrocarbon solvents.
Solvent choices for reactions involving organometallics of the alkali metals are critical. For example, nBuLi is decomposed by Et2O to give nBuH, C2H4 and LiOEt.
Alkali metal organometallics are extremely reactive and must be handled in air- and moisture-free environments; NaMe, for example, burns explosively in air.
Lithium alkyls are polymeric both in solution and in the solid state.
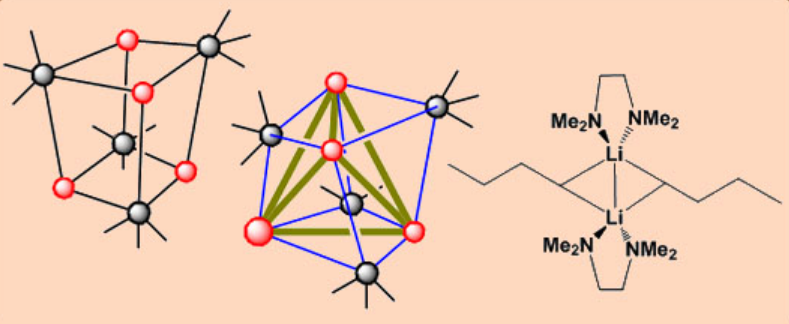
NMR is very useful in understanding the solution structures; 6Li (I = 1), 7Li (I = ½), 13C (I = ½)
The structures of (tBuLi)4 and (MeLi)4 are similar. nBuLi when mixed with TMEDA, gives a polymeric chain. TMEDA link cubane units together through the formation of Li-N bonds.
Alkyllithium compounds are soluble in organic solvents whereas Na and K salts are insoluble, but are solubilized by the chelating ligand TMEDA. Addition of TMEDA may break down the aggregates of lithium alkyls to give lower nuclearity complexes. E.g. [nBuLi.TMEDA]2
However, detailed studies have revealed that the system is far from simple, and it is possible to isolate crystals of either [nBULi.TMEDA]2 or [(nBuLi)4.TMEDA]∞.
In the case of (MeLi)4, the addition of TMEDA does not lead to cluster breakdown, and the X-ray structure confirms the composition (MeLi)4.2TMEDA, the presence of both tetramers and the amine molecules in the crystal lattice.
N. D. R. Barnett et al. J. Am. Chem. Soc., 1993, 115, 1573.
Lithium alkyls and aryls are very useful reagents in organic synthesis and also in making corresponding carbon compounds of main group elements.
Lithium alkyls are important catalysts in the synthetic rubber industry for the stereospecific polymerization of alkenes.


