2.1: General Methods of Preparation
- Page ID
- 172724
Learning Objectives
In this section you will learn the following
- Various synthetic methodologies to make M—C bonds.
- How to choose an appropriate synthetic method.
- Reaction conditions and the role of solvents
An organometallic compound contains one or more metal-carbon bonds.
Synthesis
General Methods of Preparation
Most organometallic compounds can be synthesized by using one of four M-C bond forming reactions of a metal with an organic halide, metal displacement, metathesis and hydrometallation.
a. Reaction with metal and transmetallation
The net reaction of an electropositive metal M and a halogen-substituted hydrocarbon is
\[\ce{ 2 M} + \ce{R X} (\text{alkyl or aryl}) \ce{-> MR + MX} \nonumber \]
For example
\[\ce{ 8 Li + 4CH3Cl -> Li4(CH3)4 + 4LiCl} \nonumber \]
\[\ce{ Mg + CH3Br -> CH3MgBr (organometal\:halide\:with\:Mg, Al, Zn)} \nonumber \]
If, one metal atom takes the place of another, it is called transmetallation
\[\ce{ M + M'R -> M' + MR} \nonumber \]
\[\ce{ 2Ga + 3CH3-Hg-CH3 -> 3Hg + 2Ga(CH3)3} \nonumber \]
Transmetallation is favorable when the displacing metal is higher in the electrochemical series than the displaced metal.
b. Metathesis
The metathesis of an organometallic compound MR and a binary halide EX is a widely used synthetic route in organometallic chemistry.
\[\ce {MR + EX -> ER + MX} \nonumber \]
\[\ce {Li4(CH3)4 + SiCl4 -> 4LiCl + Si(CH3)4} \nonumber \]
\[\ce {Al2(CH3)6 + 2BF3 -> 2AlF3 + 2B(CH3)3} \nonumber \]
Metathesis reaction can frequently be predicted from electronegativity or hard and soft acid-base considerations.
Hydrocarbon groups tends to bond to the more electronegative element; the halogen favors the formation of ionic compounds with the more electropositive metal.
In brief, the alkyl and aryl group tends to migrate from the less to the more electronegative element [χ = electronegativity].
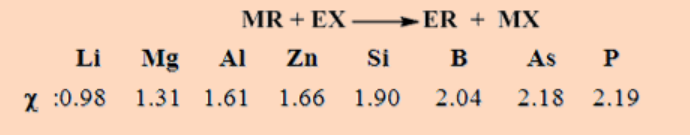
When the electronegativities are similar, the correct outcome may be predicted, with care*, by considering the combination of the softer element with organic group and harder element with fluoride or chloride.
*An insoluble product or reactant may change the outcome, e.g.;
\[\ce{SnPh4(THF) + HgBr2(THF) -> HgPhBr(s) + PhSnBr (THF)} \nonumber \]
HgPhBr turns out to be insoluble in THF
Metathesis reactions involving the same central element are often referred to as redistribution reactions.
\[\ce{SiCl4 + SiMe4 -> Me3SiCl + Me2SiCl2 + ...} \nonumber \]
\[\ce{3GeCl4 + 2AlMe6 -> 3GeMe4 + 4AlCl3} \nonumber \]
Al is more electropositive than Ge, this reaction occurs as it is thermodynamically favorable.
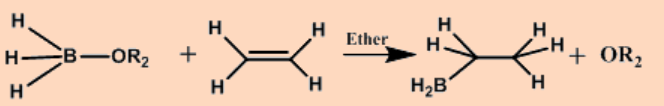
c. Hydrometallation
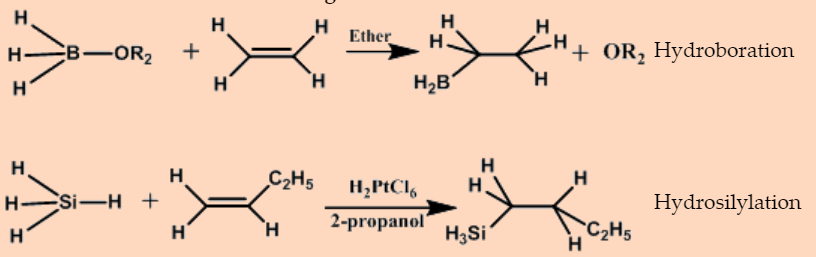
The net outcome of the addition of a metal hydride to an alkene is an alkylmetal compound.
\[\ce{EH + H2C=CH2 -> E-CH2-CH3} \nonumber \]
The reaction is driven by the high strength of E-C bond relative to that of most E-H bonds, and occurs with a wide variety of compounds that contain E-H bonds.
Hydroboration
Hydrosilylation
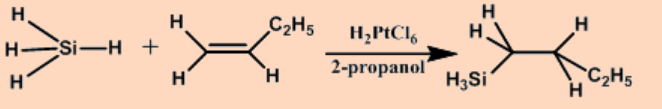
Ionic and electron-deficient compounds of Group 1, 2
Organometallic derivatives of all Group 1 metals are known. Amongst, the alkyllithium compounds are most thoroughly studied and useful reagents.
Many of them are commercially available.
MeLi is generally handled in ether solution, but RLi compounds with longer chains are soluble in hydrocarbons.
Commercial preparation:
\[\ce{M + RX -> MR (often \: contaminated \: with \: halide)} \nonumber \]
The best method would be:
\[\ce{HgR2 + 2Li -> 2LiR + Hg} \nonumber \]
MeLi exists as a tetrahedral cluster in the solid state and in the solution. Many of its higher homologs exist in solution as hexamers or equilibrium mixture of aggregates ranging up to haxamers.
The larger aggregates can be broken down by Lewis bases, such as, TMEDA.
Common organolithium compounds have one Li per organic group.
Several polylithiated organic molecules containing several lithium atoms per molecule are known.
The simplest example is Li2CH2, which can be prepared by the pyrolysis of MeLi which crystallizes in a distorted antifulorite* structure. However, the finer details of the orientation of the CH2 groups are yet to be established.
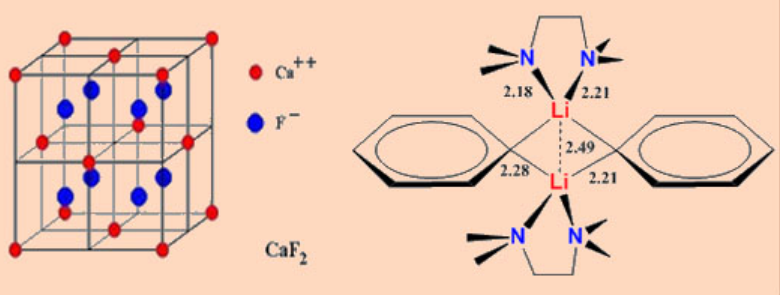
*the antifluorite structure is the inverse of the fluorite structure in which the locations ofcations and anions are reversed. Look into the structures of CaF2 (fluorite structure) and K2O (antifluorite structure). An fcc array of cations and all the tetrahedral holes are filled with anions.
Radical anion salts
Sodium naphthalide is an example of an organometallic salt with a delocalized radical anion, C10H8-.
Such compounds are readily prepared by reacting an aromatic compound with an alkali metal in a polar aprotic solvent.
Naphthalene dissolved in THF reacts with Na metal to produce a dark green solution of sodium naphthalide.
\[\ce{Na(s) + C10H8(THF) -> Na[C10H8](THF)} \nonumber \]
EPR spectra show that the odd electron is delocalized in an antibonding orbital of C10H8.
Formation of radical anion is more favorable when the π of LUMO of the arene is low in energy.
Simple MOT predicts that the energy of LUMO decreases steadily on going from benzene to more extensively conjugated hydrocarbons.
Sodium naphthalide and similar compounds are highly reactive reducing agents.
They are preferred to sodium because unlike sodium, they are readily soluble in ethers.
The resulting homogeneous reaction is generally faster and easier to control than a heterogeneous reaction between one reagent in solution and pieces of sodium metal, which are often coated with unreactive sodium oxide or with insoluble reaction products.
The additional advantage is that by proper choice of the aromatic group the reduction potential of the reagent can be chosen to match the requirements of a particular synthetic task.
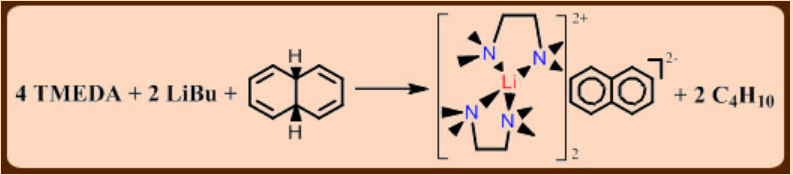
Alternative route to delocalized anion is the reductive cleavage of acidic C—H bonds by an alkali metal or alkylmetallic compound.
Example:
Problems:
1. Classify the following reactions into, (i) hydrometallation, metal displacement, metathesis OR transmetallation reactions; (ii) give an example for each case in the form of a balanced chemical equation.
Solution
a. \[\ce{M + Mx’R -> M’ + MR} \nonumber \] ….Transmetallation
e.g.: \[\ce{2Ga + 3CH3-Hg-CH3 -> 3Hg + 2Ga(CH3)3} \nonumber \]
b. \[\ce{MR + EX → ER + MX} \nonumber \] ….Metathesis
e.g.: \[\ce{Li4(CH3)4 + SiCl4 -> 4LiCl +Si(CH3)4} \nonumber \] or \[\ce{Al2(CH3)6 + 2BF3 -> 2AlF3 + 2B(CH3)3} \nonumber \]
c. \[\ce{EH + H2C=CH2 -> E—CH2—CH3 } \nonumber \] ….Hydrometallation
e.g.:
Problems:
2. For each of the following compounds, indicate those that may serve as
(1) a good carbanion nucleophile reagent,
(2) a mild Lewis acid,
(3) a mild Lewis base at the central atom,
(4) a strong reducing agent. (A compound may have more than one of these properties)
(a) Li4(CH3)4, (b) Zn(CH3)2, (c) (CH3)MgBr, (d) B(CH3)3, (e) Al2(CH3)6, (f) Si(CH3)4, (g) As(CH3)3.
Solution
- (MeLi)4 - good carbanion nucleophile and strong reducing agent
- ZnMe2 - reasonable carbanion nucleophile, mild Lewis acid, reducing agent
- MeMgBr - good carbanion nucleophile
- BMe3 - mild Lewis acid
- Al2Me6 - good carbanion nucleophile, strong reducing agent
- SiMe4 - mild Lewis acid
- AsMe3 - mild Lewis base


