8.1: Catalytic reactions
- Page ID
- 125420
Catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Therefore they are crucially important in chemical industry, exhaust gas treatment and other chemical reactions. While the chemical essence of catalysis is obscure, practical catalysts have been developed based on the accumulation of empirical knowledge. However, while gradually we have come to understand the mechanisms of homogeneous catalysis through the development of inorganic chemistry, our understanding of surface reactions in solid catalysts is also deepening.
(a) Homogeneous catalysis
The chemistry of catalysts that are soluble in solvents has developed remarkably since the epoch-making discovery (1965) of the Wilkinson catalyst, [RhCl(PPh3)3]. This complex is a purplish red compound which forms by heating RhCl3 • 3 H2O and PPh3 under reflux in ethanol. When dissolved in an organic solvent, this complex is an excellent catalyst for hydrogenation of unsaturated hydrocarbons by H2 at ambient temperatures and pressures to form saturated hydrocarbons, and hydroformylation reactions of olefins with H2 and CO to form aldehydes.
In the past, the mechanism of catalytic reactions were generally not very clear. Before the Wilkinson catalyst, the Reppe process, which oligomerize aceylenes or the Ziegler-Natta catalysts that polymerize olefins and dienes, had been discovered and detailed studies on homogeneous catalysis had been conducted from the viewpoint of the chemistry of complexes. Consequently, catalytic reactions are now established as a cycle of a combination of a few elementary steps that occur on the metals of catalyst complexes.
Coordination and dissociation
There must be a process in which reactants such as olefins are activated and react with other reactants after being coordinated to the central metal of a complex, and they dissociate from the metal as products.
Oxidative addition
Oxidative addition is one among a few key elementary reactions of metal complexes. This is a reaction of such compounds as alkali metal halides, RX, acids, HX or dihydrogen, H2, to the metal in a complex which then dissociate into R and X, H and X, or H and H, which are bonded to the metal as two fragment anions. If other ligands on the start complex are not removed, the coordination number increases by two. As alkyl, halogen, and hydride ligands are more electronegative than the central metal, they are regarded as formally anionic ligands after coordination. Therefore, the oxidation number of the central metal increases after an addition reaction. As it is an addition reaction accompanied by oxidation of the central metal, it is called oxidative addition.
For example, in the addition reaction of an alkyl halide to a tetra-coordinate iridium(I) complex [IrCl(CO) (PPh3)2],
\[[Ir^{I} Cl (CO) (PPh_{3})_{2}] + RI \rightarrow [Ir^{III}(Cl)(I)(R)(CO)(PPh_{3})_{2}]\]
iridium becomes hexa-coordinate and undergoes two-electron oxidation from +1 to +3. Since a neutral RI molecule is added, there must be no change in the charge of the whole complex, and if an alkyl and iodine are anions, the oxidation number of the central metal should increase by 2. Similar change occur when two hydride ligands are formed as the result of the addition of dihydrogen.
The reverse reaction is called reductive elimination. Both oxidative and reductive reactions are very important as elementary steps in the mechanism of homogeneous catalysis involving hydrocarbons and dihydrogen.
Exercise \(\PageIndex{1}\)
How does the oxidation number of rhodium change with reductive elimination of dihydrogen from [RhCl(H)2(PPh3)2 (Sol)]?
- Answer
-
It changes to Rh(I) from Rh(III).
Insertion reaction
In the reaction of an alkyl or hydride ligand to shift to a carbonyl or olefin ligand coexisting on the central metal, the resultant complex appears as if a carbonyl or an olefin has inserted between the M-R or M-H bond. This is called an insertion reaction.
Reaction of a coordinated ligand
This is the process in which a coordinated reactant reacts to form a product. By coordinating to a metal, the reactants take geometrically and electronically suitable conformations. It is the basis of catalyst design to control these reaction conditions.
Since a reaction is repeated while the complex used as a catalyst remains unchanged by forming a cycle of reactions, the reactants/complex ratio is very small, coinciding with the definition of a catalyst. The catalytic cycle in hydrogenation of ethylene is illustrated in Figure \(\PageIndex{1}\).
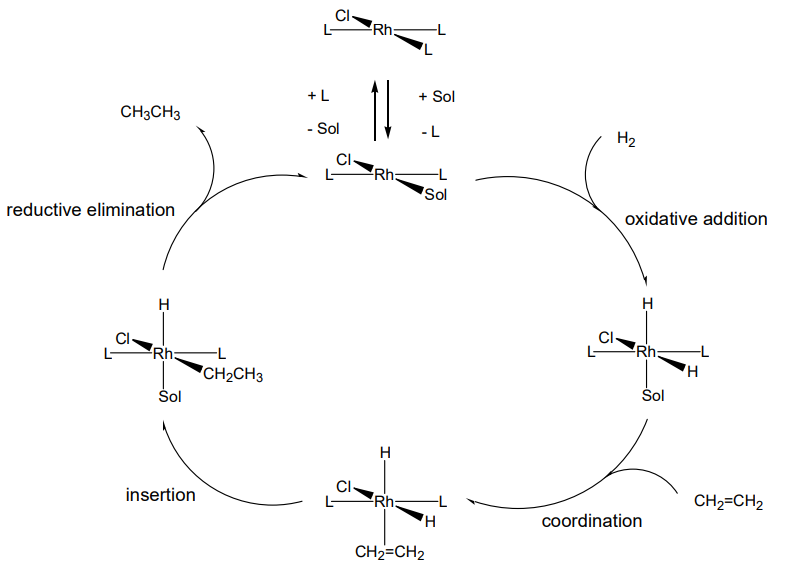
If the triphenylphosphine ligand P(C6H5)3 in the Wilkinson catalyst is replaced by an optical active phosphine, asymmetric hydrogenation is realized. Asymmetrical catalysis equivalent to enzyme reactions have been developed by skillful design of asymetrical ligands. In particular, the asymmetric induction of binaphtyldiphosphine (BINAP) has attracted attention.
(b) Solid state catalysis
A solid catalyst is also called a heterogeneous catalyst, and promotes the reaction of reactants in gaseous or liquid phases in contact with a solid material. Since adsorption of reactants on the catalyst surface is the initial step, a large surface area is required for good efficiency of catalysis. Polyphase systems, which carry active catalysts on materials such as zeolites with small pores of molecular sizes, and gamma alumina and silica gel with large surface area, are often used.
Previously, solid state catalysis was explained as arising from a mysterious activation of reactants due to adsorption, but it has become increasingly clear that catalysis is ascribable to surface chemical reactions. Namely, the action of solid state catalysts depends on activation of reactants by surface acids or bases, and by coordination to the metal surface. It is possible to observe these interactions using various spectroscopies (infrared spectroscopy, EXAFS (extended X-ray absorption fine structure), electronic spectra), electron microscopy, or STM (scanning tunnelling microscopy).
Since mechanisms of homogeneous catalysis have been clarified considerably, solid surface reactions can also be analyzed by introducing concepts such as “surface complexes” or “surface organometallic compounds”. However, unlike homogeneous catalysis, in which only one or a few metal centers participate, many active sites are involved in solid state catalysis. Since surface homogeneity and reproducibility are difficult to maintain, major parts of reaction mechanisms are obscure even for such simple reactions as ammonia synthesis.
During the direct production of ammonia from dinitrogen and dihydrogen, reactions occur using iron catalysts containing alkali metal or alkaline earth metal oxides as activators at high temperatures (about 450 °C) and under high pressures (about 270 atm). Prior to the epoch-making discovery of this process by F. Haber (1909), all nitrogen compounds came from natural resources. The realization of this discovery has had an immeasurable influence upon chemical industries, as ammonia is indispensable to the manufacture of fertilizers, gunpowder and other inorganic compounds containing nitrogen. In recognition of this, a Nobel Prize was awarded to F. Haber for this invention (1918). A huge volume of research on the elucidation of the reaction mechanism of ammonia synthesis has been performed up until the present, because the reaction of dinitrogen and dihydrogen on iron catalysts is a good model of solid state catalysis.

