6.4: Organometallic Chemistry of d Block Metals (Part 1)
- Page ID
- 125408
The organometallic chemistry of transition metals is comparatively new. Although an ethylene complex of platinum called Zeise's salt, K[PtCl3(C2H4)], tetracarbonylnickel, Ni(CO)4, and pentacarbonyliron, Fe(CO)5, which today are classified as organometallic compounds, were prepared in the 19th century, their bonding and structures were unknown. The research of W. Hieber and others on metal carbonyl compounds was important in the 1930s, but the results of these studies were limited because of the underdeveloped techniques of structural analyses available at the time.
The discovery of ferrocene, Fe(C5H5)2, in 1951 was epoch-making for the chemistry of this field. The very unique bonding mode of this complex became clear by means of single crystal X-ray structural analysis, NMR spectra, infrared spectra, etc., and served as a starting point for subsequent developments in the field. It was a major discovery that ferrocene exhibited very high thermal stability in spite of the general view that the transition metal-carbon bonds were very unstable. It was also clearly demonstrated that the compound had a sandwich structure in which the five carbon atoms of the cyclopentadienyl groups bonded simultaneously to the central metal iron. While the various coordination modes of hydrocarbon ligands were determined one after another, the industrial importance of organometallic compounds of transition metals increased with the discoveries of olefin polymerization catalysts (Ziegler catalyst), homogeneous hydrogenation catalysts (Wilkinson catalyst), and development of catalysts for asymmetric synthesis, etc. The Nobel prize awarded to K. Ziegler, G. Natta (1963), E. O. Fischer, and G. Wilkinson (1973) was in recognition of this importance.
According to the definition of an organometallic compound, at least one direct bond between a metal and a carbon atom should exist, but CN complexes etc. with no organometallic character are usually excluded from organometallic compounds. Metal carbonyl compounds are organometallic in various aspects of their bonding, structure and reactions, and they are a good model system for understanding of the essence of transition metal organometallic chemistry.
(a) Metal carbonyl compounds
Binary metal carbonyl compounds that consist only of a metal and CO ligands are usually prepared by the direct reaction of the powder of a highly reactive metal and carbon monoxide, or by the reduction of a metal salt to zero valance followed by reaction with high-pressure carbon monoxide. However, tetracarbonylnickel, first discovered at the end of the 19th century, forms by the reaction of nickel metal and carbon monoxide under atmospheric pressure and at room temperature. The preparation of other metal carbonyl compounds, on the other hand, requires high temperatures and high pressures.
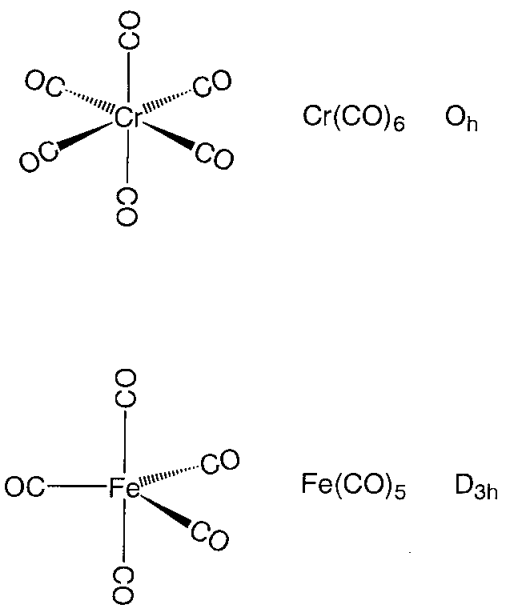
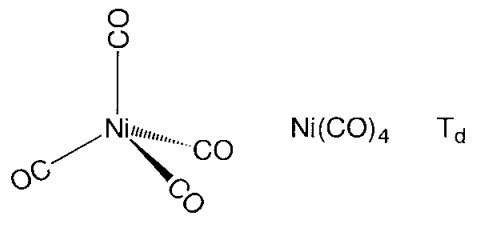
Mononuclear metal carbonyl compounds take highly symmetric polyhedral coordination structures. Hexa-coordinate chromium, molybdenum, and tungsten hexacarbonyl, M(CO)6, assume a regular octahedral, penta-coordinate pentacarbonyliron, Fe(CO)5, a triangular bipyramid, and tetracarbonylnickel, Ni(CO)4, a regular tetrahedron coordination structure (Figure \(\PageIndex{14}\)). The carbon atoms of carbonyl ligands coordinate to the metal, and the CO moieties are oriented along the direction of the metal-carbon axis. Binuclear metal carbonyl Mn2(CO)10 has an Mn-Mn bond joining two square pyramidal Mn(CO)5 parts. In Fe2(CO)9, two Fe(CO)3 sub-units are bridged by three CO ligands, and in Co2(CO)8, two Co(CO)3 sub-units are connected by both three CO bridges and a Co-Co bond.
There are a number of cluster metal carbonyl compounds with metal-metal bonds joining three or more metals, and terminal CO, \(\mu\)-CO (a bridge between two metals), and \(\mu_{3}\)-CO (a bridge capping three metals) are coordinated to the metal frames (refer to Section 6.3 (f)). Many cluster carbonyls are formed by a pyrolysis reaction of mononuclear or binuclear carbonyl compounds. Typical metal carbonyl compounds and their properties are shown in Table \(\PageIndex{4}\).
| 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|
| 4 | V(CO)6 Black solid d.70 |
Cr(CO)6 White solid d.130 |
Mn2(CO)10 Yellow solid mp 154 |
Fe(CO)5 Yellow liquid bp 103 |
Co2(CO)8 Red solid mp 51 |
Ni(CO)4 Colorless liquid bp 42.1 |
| 5 | Mo(CO)6 White solid sublime |
Tc2(CO)10 White solid mp 160 |
Ru3(CO)12 Orange solid d.150 |
Rh6(CO)16 Black solid d.220 |
||
| 6 | W(CO)6 White solid sublime |
Re2(CO)10 White solid mp 177 |
Os3(CO)12 Orange solid mp 224 |
Ir4(CO)12 Yellow solid d.220 |
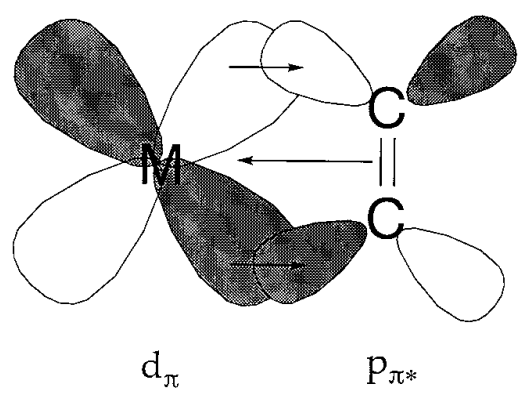
Back donation
A metal carbonyl compound consists of carbon monoxide coordinated to a zero valent metal. For a long time, it had been unclear why such bonding was possible, let alone stable at all. The belief that normal coordination bonds were formed by the donation of electrons from highly basic ligands to a metal formed the basis of the coordination theory of A. Werner. Because the basicity of carbon monoxide is very low, and transition metal-carbon bonds are generally not very stable, a suitable explanation for the stability of metal carbonyl compounds was sought. If the shape and symmetry of the metal d orbital and of the CO \(\pi\) (antibonding) orbital for the carbon-oxygen bond are suitable for overlap, a bonding interaction between the metal and carbon is expected. The bonding scheme shown in Figure \(\PageIndex{15}\) was proposed from this point of view. The mechanism by which electrons are donated to the vacant carbon monoxide \(\pi^{*}\) orbital from the filled metal d orbital is called back donation. Since accumulation of superfluous electrons on a low oxidation state metal atom is prevented, back-donation leads to the stabilization of the M-C bond.
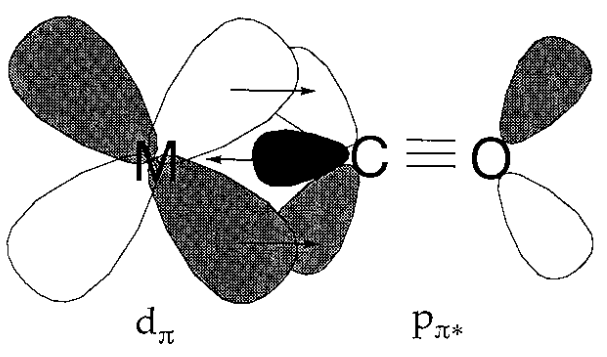
Fig 6.15 - Back donation in metal carbonyls.
A rise in the order of the metal - carbon bond is reflected in the increase of the M-C, and decrease of the C-O, stretching frequencies in vibrational spectra. Infrared spectra are useful because carbonyl frequencies are easily detectable. The lowering of the oxidation state of a metal by the flow of negative charge from its coordinated ligands is reflected in the reduction of the C-O stretching frequencies.
(b) Hydrocarbon complexes
An organometallic compound is one which has metal-carbon bonds, and between one and eight carbon atoms in a hydrocarbon ligand bond to a metal. Hapticity describes the number of atoms in a ligand that have direct coordinative interaction with the metal and the number is added to \(\eta\). An example is \(\eta^{5}\) (pentahapto)-cyclopentadienyl (Table \(\PageIndex{5}\)).
A ligand that donates an odd number of electrons to a metal is formally a radical and it is stabilized by bonding to the metal. A ligand that donates an even number of electrons to a metal is generally a neutral molecule and it is stable even if it is not bonded to the metal. Carbene or carbyne ligands are exceptions to this rule. The chemical formula of an organometallic compound is expressed in many cases without using the square brackets [ ] usual for such a complex, and we shall follow this convention in this book.
| Name | Hapticity | Number of electrons | Example |
|---|---|---|---|
| Alkyl | \(\eta^{1}\) | 1 | W(CH3)6 |
| Alkylidene | \(\eta^{1}\) | 2 | Cr(CO)5{C(OCH3)C6H5} |
| Alkene | \(\eta^{2}\) | 2 | K[PtCl3(C2H4)] |
| \(\pi\)-allyl | \(\eta^{3}\) | 3 | Ni(\(\eta^{3}\)-C3H5)2 |
| Diene | \(\eta^{4}\) | 4 | Fe(CO)3(\(\eta^{4}\)-C4H6) |
| Cyclopentadienyl | \(\eta^{5}\) | 5 | Fe(\(\eta^{5}\)-C5H5)2 |
| Arene | \(\eta^{6}\) | 6 | Cr(\(\eta^{6}\)-C6H6)2 |
| Tropylium | \(\eta^{7}\) | 7 | V(CO)3(\(\eta^{8}\) -C7H7) |
| Cyclooctatetraene | \(\eta^{8}\) | 8 | U(\(\eta^{8}\) -C8H8)2 |
Exercise \(\PageIndex{4}\)
Describe the difference between cyclopentadiene and cyclopentadienyl ligands.
- Answer
-
The chemical formula of cyclopentadiene is C5H6 and it is bonded to a metal as a \(\eta^{2}\) or \(\eta^{4}\) ligand. The chemical formula of cyclopentadienyl is C5H5 and it is bonded to a metal as a \(\eta^{1}\), \(\eta^{3}\), or \(\eta^{5}\) ligand.
Alkyl ligands
Alkyl or aryl transition metal compounds have M-C single bonds. In spite of many attempts over most of the course of chemical history, their isolation was unsuccessful and it was long considered that all M-C bonds were essentially unstable. Stable alkyl complexes began to be prepared gradually only from the 1950s. Cp2ZrCl(Pr),WMe6, CpFeMe(CO)2, CoMe(py)(dmg)2, (dmg = dimethylglyoximato), IrCl(X)(Et)(CO)(PPh3)2, NiEt2(bipy), PtCl(Et)(PEt3)2 are some representative compounds. Among various synthetic processes so far developed, the reactions of compounds containing M-halogen bonds with main-group metal-alkyl compounds, such as a Grignard reagent or an organolithium compound, are common synthetic routes. Especially vitamin B12, of which D. Hodgkin (1964 Nobel Prize) determined the structure, is known to have a very stable Co-C bond. Metal alkyl compounds which have only alkyl ligand, such as WMe6, are called homoleptic alkyls.
It is gradually accepted that a major cause of the instability of alkyl complexes is the low activation energy of their decomposition rather than a low M-C bond energy. The most general decomposition path is \(\beta\) elimination. Namely, the bonding interaction of a hydrocarbon ligand with the central transition metal tends to result in the formation of a metal hydride and an olefin. Such an interaction is called an agostic interaction. Although an alkyl and an aryl ligand are 1-electron ligands, they are regarded as anions when the oxidation number of the metal is counted. The hydride ligand, H, resembles the alkyl ligand in this aspect.
\(\pi\) allyl complexes
If an allyl group, CH2=CH-CH2-, is bonded to a metal via a carbon atom, it is a 1-electron ligand like an alkyl group. If the double bond delocalizes, three carbon atoms bond to the metal simultaneously as a 3-electron ligand. This is also an odd electron and formally anionic ligand and is stabilized by being coordinated to the metal.
Pd(C3H5)(Ac)(PPh3), Co(C3H5)3, etc are well-known examples. Since \(\eta^{1}\), \(\eta^{2}\), and \(\eta^{3}\) coordination modes are possible in the catalytic reactions of unsaturated hydrocarbons, various reactions occur.
\(\pi\) cyclopentadienl complexes
The cyclopentadie nyl ligand, C5H5, is abbreviated as Cp. C5Me5, in which the hydrogen atoms of Cp are replaced with methyl groups, is a useful ligand called Cp star and is denoted by Cp*. Ferrocene, Cp2Fe, is a very stable orange-colored iron compound in which two cyclopentadienyl groups are bonded to iron. It was discovered independently in two laboratories, but the discoverers proposed incorrect structures. The correct structure was clarified by the group of G. Wilkinson, who won a Nobel Prize (1973). The preparation of ferrocene is usually carried out according to the following reaction path:
\[ \ce{2 C_{5} H_{6} + 2 Na \rightarrow 2 Na(C_{5}H_{5}) + H_{2}}\]
\[\ce{FeCl_{2} + 2 Na(C_{5}H_{5}) \rightarrow Fe(C_{5}H_{5})_{2} + 2 NaCl}\]
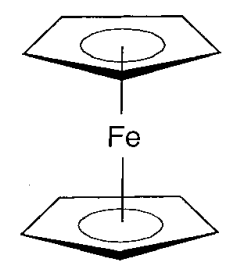
Single crystal X-ray structure analysis showed that the structure of ferrocene is an iron atom sandwiched between two C5H5 rings (Figure \(\PageIndex{16}\)). Five carbon atoms bond to the iron simultaneously in ferrocene, and unsaturated C-C bonds are delocalized in the five-membered rings. Since this kind of bond was not known before, it aroused interest, many derivative compounds were prepared, and a wide range of chemistry has since been studied (Table \(\PageIndex{6}\)).
| 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|
| 4 | Cp2TiCl2 Red mp 230 |
Cp2V Black mp 167 |
Cp2Cr Scarlet mp 173 |
Cp2Mn Brown mp 193 |
Cp2Fe Orange mp 174 |
Cp2Co Black mp 173 |
Cp2Ni Green d.173 |
| 5 | Cp2ZrCl2 White mp 248 |
Cp2NbCl2 Brown |
Cp2MoCl2 Green d.270 |
Cp2TcH Yellow mp 150 |
Cp2Ru Yellow mp 200 |
||
| 6 | Cp2HfCl2 White mp 234 |
Cp2TaCl2 Brown |
Cp2WCl2 Green d.250 |
Cp2ReH Yellow mp 161 |
Cp2Os White mp 229 |
The cyclopentadienyl ligand is a 5-electron and formally anionic ligand. If only one of the five carbon atoms is bonded to a metal, it is a 1-electron ligand like an alkyl group. It becomes a 3-electron ligand in rare cases and coordinates to a metal as a \(\pi\)-allyl system that extends over 3 carbon atoms. The Cp group of ferrocene has reactivity analogous to that of aromatic compounds. Since the Cp group has played a significant role as a stabilizing ligand to realize the preparation of new compounds with new metal-ligand bonding modes, it can reasonably be claimed that this ligand has made the greatest contribution to organometallic chemistry of any other ligand. Although two Cp rings are bonded to the metal in parallel in ferrocene, Cp2TiCl2 and Cp2MoH2 have bent Cp ligands and they are called bent-sandwich compounds.
Olefin complexes
Zeise's salt, K[PtCl3(C2H4)], is the oldest known organometallic compound and was synthesized and analyzed in ca. 1825 by Zeise, although its coordination structure was assumed only in 1954 and confirmed by the neutron diffraction in 1975. The mode of coordination of an olefin to a transition metal is described by the Dewar-Chatt-Duncanson model and the bond between the metal and olefin is stabilized by the contribution of d\(\pi\)-p\(\pi^{*}\) back donation. An olefin is a 2-electron ligand and there are many olefin complexes in which the central metal is in a relatively low oxidation state. Dienes or trienes with two or more double bonds coordinate to a metal as 4-electron or 6-electron ligands. Fe(CO)3(C4H6) and Ni(cod)2, in which a butadiene or cyclooctadienes (cod) are coordinated to the metal, are well known examples. Since cyclooctadienes are easily eliminated from Ni(cod)2, it is conveniently used for generating atomic, zero valent nickel. This complex is sometimes called naked nickel.
Arene complexes
Aromatic compounds are 6-electron donors that coordinate to transition metals in the \(\eta^{6}\) coordination mode with six carbon atoms. Bisbenzenechromium, Cr(C6H6)2, is a typical example of such a compound. The compound is prepared by reducing chromium chloride in benzene and it has a sandwich structure in which a chromium atom is insertedb etween two benzene rings. When a benzene ligand is replaced by three carbonyls, Cr(CO)3(C6H6) is obtained.
18 electron rule
Counting valence electrons is of utmost importance in chemistry. Changes in the number of valence electrons has a profound influence on the bonding, structure, and reactions of a compound. Since both the metal and organic moieties are involved in organometallic compounds, counting the number of electrons becomes complicated. Hydrocarbyl ligands are classified as either neutral molecules coordinating to the metal or radicals bonding to the metal, and the radicals, such as alkyls and cyclopentadienyl, are generally called anionic ligands. Transfer of one electron from the metal to the radical ligand makes the ligand formally anionic. However, it is less confusing to consider that both the metal and the ligands are neutral when counting the number of valence electrons. The numbers of donor electrons in typical carbon ligands from this viewpoint are listed in Table \(\PageIndex{5}\). It is important to note that even in the same ligand, the number of donor electrons supplied by the ligand differs depending upon the number of ligating atoms that have coordinative interactions with the metal. For example, 1, 3 or 5 electrons can be donated from a cyclopentadienyl ligand, depending on the type of coordinative interactions with the metal.
When the total number of valence electrons of the metal and ligands is 18, a transition metal organometallic compound usually has high thermal stability. For example Cr(CO)6, Fe(CO)5, Ni(CO)4, Fe(C5H5)2, Mo(C6H6)(CO)3 etc. satisfy the 18 electron rule, but the monomeric parts of Mn2(CO)10, Co2(CO)8 or [Fe(C5H5)(CO)2]2 have only 17 electrons and the extra electron comes from the partner metal by forming a metal-metal bond. Unlike the 8 electron rule in main group compounds, applicability of the 18 electron rule is limited. That is to say, it is a sufficient condition but compounds with high thermal stability are not necessarily 18 electron compounds.
Although there are many Group 6 (chromium group) through Group 9 (cobalt group) organometallic compounds with carbonyl or cyclopentadienyl ligands that satisfy the 18 electron rule, many compounds of the early transition metals (Group 3 - 5) and Group 10 (nickel group) fail to conform to this rule. For example, W(CH3)6 (12e), TiCl2(C5H5)2 (16e), and IrCl2(CO)(PPh3)2 (16e), V(CO)6 (17e), Co(C5H5)2 (19e), Ni(C5H5)2 (20e), etc. do not satisfy the 18 electron rule. However, the 18 electron rule provides useful clues as to the bonding modes present in a given complex. For example, Fe(C5H5)2(CO)2 with two pentahapto cyclopentadienyl ligands formally has 22 electrons but if one of the ligands is monohapto, the compound has 18 electrons. Structural analysis has shown that this is the actual coordination of this complex.
Exercise \(\PageIndex{5}\)
Calculate the valence electron number of CpMn(CO)3.
- Answer
-
They are a total of 18 electrons from Mn (7), Cp(5) and three CO(6).
(c) Phosphine complexes
Tertiary phosphines, PX3, are very useful as stabilization ligands in transition metal complexes and they coordinate to the metals in relatively high to low oxidation states. Phosphines are frequently used as carbonyl or cyclopentadienyl ligands in the chemistry of organometallic complexes. PX3 are Lewis bases and coordinate to the metal using the lone pair on phosphorus and show \(\pi\)-acidity when carrying substituents X including Ph, Cl, or F that have strong electron accepting properties. The electronic flexibility of PX3 is the reason it forms so many complexes. Generally, the π-acidity becomes smaller in the order PF3 > PCl3 > PPh3 > PR3. Triphenylphosphine and triethylphosphine are typical substituted phosphines. The tertiaryphosphine complexes mainly of metal halides are listed in Table \(\PageIndex{7}\). Manganese, Mn, and the early transition metals form very few phosphine complexes.
| 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|
| 4 | [TiCl4(PPh3)2] | [VCl3(PMe Ph2)2] |
[CrCl2 (dmpe)2] |
[Mn(CO)4 (PPh3)] |
[FeCl2 (PPh3)2] |
[CoCl2 (PPh3)2] |
[NiCl2(PEt3)2] | [CuBr(PEt3)]4 |
| 5 | [ZrCl4(dppe)] | [NbCl4(PEt Ph2)2] |
[MoCl3(PMe Ph2)3] |
[TcCl3(PMe2 Ph)3] |
[RuCl2 (PPh3)3] |
[RhCl(PPh3)3] | [PdCl2(P Ph3)2] |
[AgCl(PPh3)] |
| 6 | [HfCl4(dppe)] | [TaCl4(PEt3)2] | [WCl4(PPh3)2] | [ReCl3(PMe2 Ph)3] |
[OsCl3 (PPh3)3] |
[IrCl3(PPh3)3] | [PtCl2(P Ph3)2] |
[AuCl(PPh3)] |
Many derivatives can be prepared by substituting the halogens of the phosphine complexes. A number of the complexes of polydentate phosphines with more than two coordination sites, as well as those of monodentate phosphines, have been prepared, and they are used also as stabilization ligands in hydride, alkyl, dinitrogen, and dihydrogen complexes. The complexes of rhodium or ruthenium, in which optically active phosphines are coordinated, are excellent catalysts for asymmetric synthesis.

