6.2: Electronic Structure of Complexes (Part 1)
- Page ID
- 125407
It is necessary to learn a few concepts to understand the structure, spectrum, magnetism, and reactivity of complexes which depend on d electron configurations. In particular, the theory of electronic structure is important.
Ligand field theory
Ligand field theory is one of the most useful theories to account for the electronic structure of complexes. It originated in the application of the crystal field theory of ionic crystals to metal complex systems.
Six co-ordinate octahedral complexes
The five d orbitals of transition metal cations are degenerate and have equal energy.
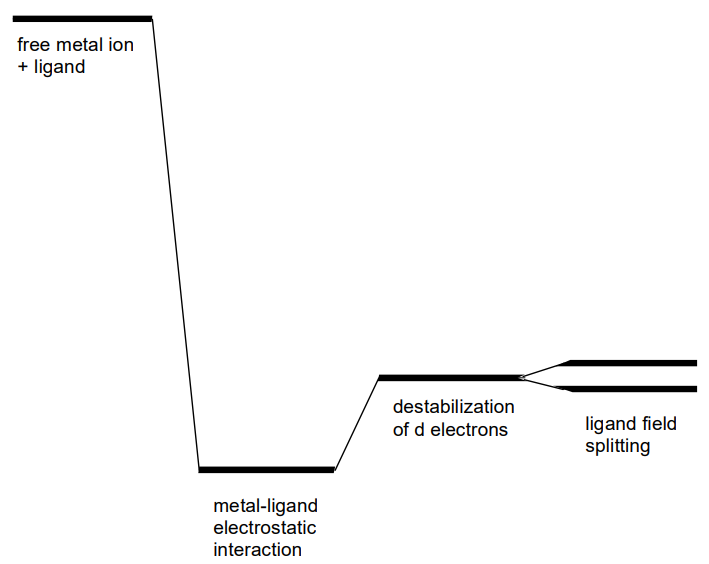
The spherical negative electric field around a metal cation results in the total energy level being lower than that of a free cation because the electrostatic interactions. The repulsive interaction between the electrons in the metal orbitals and the negative electric field destabilizes the system and compensates for the stabilization to some extent (Figure \(\PageIndex{4}\)).
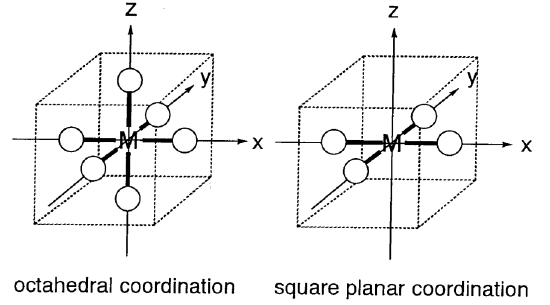
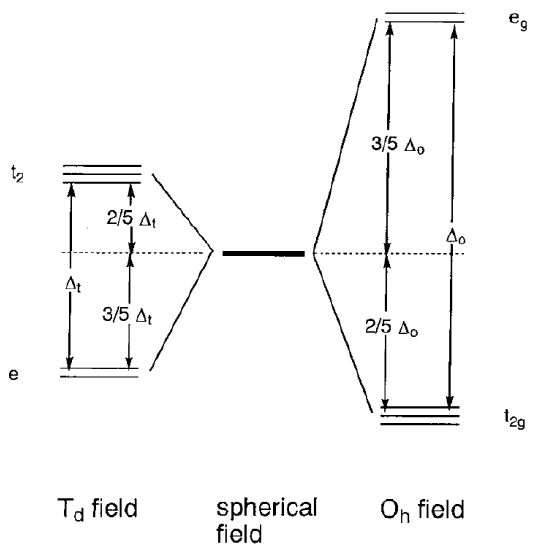
Let us assume that instead of a uniform spherical negative field, the field is generated by six ligands coordinating octahedrally to a central metal. The negative field of the ligands is called the ligand field. Negative charge, in the case of anionic ligands, or a negative end (lone pair), in the case of neutral ligands, exert a repulsive force on the metal d orbitals which is anisotropic depending on the direction of the orbitals. The position of the metal cation is taken as the origin and Cartesian coordinates are constructed (Figure \(\PageIndex{5}\)). Then, dx2-y2 and dz2 orbitals are aligned along the directions of the axes and the dxy, dyz, and dxz orbitals are directed between the axes. If ligands are placed on the axes, the repulsive interaction is larger for the eg orbitals (dx2-y2, dz2) than for the t2g orbitals (dxy, dyz, dxz), and the eg orbitals are destabilized and the t2g orbitals are stabilized to an equal extent. In the following discussion, only the energy difference between the t2g and eg orbitals is essential and the average energy of these orbitals is taken as the zero of energy. If the energy difference between the two eg and three t2g orbitals is set to \(\Delta_{o}\), the energy level of the eg orbitals is +3/5 \(\Delta_{o}\) and that of the t2g orbitals is -2/5 \(\Delta_{o}\) (Figure \(\PageIndex{6}\)). (\(\Delta_{o}\) may also be expressed as 10 Dq. In this case, the energy level of the eg orbitals is +6 Dq and that of the t2g orbitals -4 Dq.)
Transition metal ions have 0 to 10 d electrons and when the split d orbitals are filled from a lower energy level, the electron configuration t2gxegy corresponding to each ion is obtained. With the zero energy level chosen as the average energy level, the energy of the electron configuration relative to zero energy becomes
\[LFSE = (-0.4x + 0.6y) \Delta_{o}\]
This value is called the ligand field stabilization energy. The electron configuration with smaller value (taking the minus sign into consideration) is more stable. LFSE is an important parameter to explain some properties of d-block transition metal complexes.
A condition other than the orbital energy level is required to explain the filling of electrons being populated into the split t2g and eg orbitals,. Two electrons can occupy an orbital with anti-parallel spins but a strong electrostatic repulsion occurs between two electrons in the same orbital. This repulsive interaction is called pairing energy, P.
When the number of d electrons is less than three, the pairing energy is minimized by loading the electrons in the t2g orbital with parallel spins. Namely, the electron configurations arising are t2g1, t2g2, or t2g3.
Two possibilities arise when the fourth electron occupies either of the t2g or eg orbitals. The lower energy orbital t2g is favorable but occupation of the same orbital gives rise to pairing energy, P. The total energy becomes
\[-0.4 \Delta_{o} \times 4 + P = - 1.6 \Delta_{o} + P\]
If the fourth electron occupies the energetically unfavorable eg orbital, the total energy becomes
\[-0.4 \Delta_{o} \times 3 + 0.6 \Delta-{o} = - 0.6 \Delta_{o}\]
The choice of the electron configuration depends on which of the above values is larger. Therefore if \(\Delta_{o}\) > P, t2g4 is favoured and this is called the strong field case or the low spin electron configuration. If \(\Delta_{o}\) < P, t2g3eg1 is favoured and this is called the weak field case or the high spin electron configuration. A similar choice is required for d5, d6, and d7 octahedral complexes, and in the strong field case, t2g5, t2g6, or t2g6eg1 configurations are favoured, whereas in the weak field case, t2g3eg2, t2g4eg2, or t2geg2 configurations are favoured. The ligand field splitting parameter \(\Delta_{o}\) is decided by the nature of the ligands and metal, whereas the pairing energy, P, is almost constant and shows only a slight dependence on the identity of the metal.
Square planar complexes
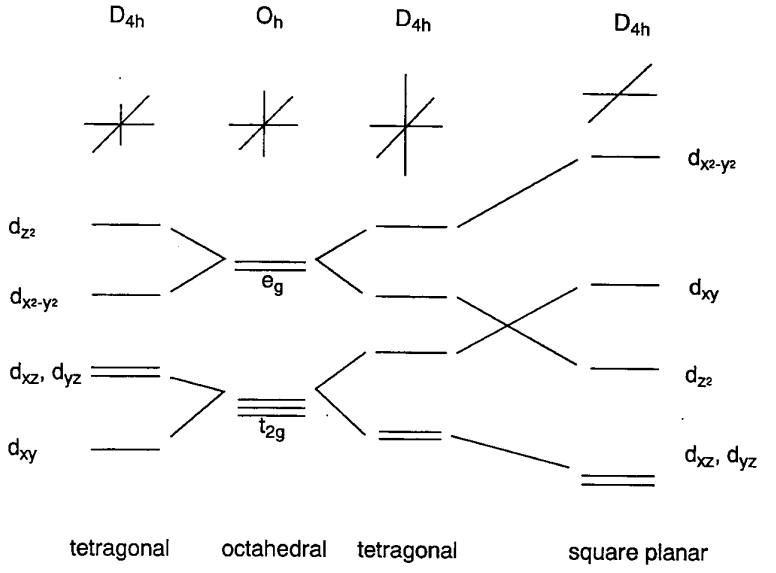
Complexes with four ligands in a plane containing the central metal are termed square planar complexes. It is easier to understand the electronic energy levels of the d orbitals in square planar complexes by starting from those for hexacoordinate octahedral complexes. Placing the six ligands along the Cartesian axes, the two ligands on the z axis are gradually removed from the central metal and finally only four ligands are left on the x,y plane. The interaction of the two z coordinate ligands with the dz2, dxz, and dyz orbitals becomes smaller and the energy levels of these ligands lower. On the other hand, the remaining four ligands approach the metal and the dx2-y2 and dxy energy levels rise as a result of the removal of the two ligands. This results in the order of the energy levels of five d orbitals being dxz, dyz < dz2 < dxy << dx2-y2 (Figure \(\PageIndex{7}\)). Rh+, Ir+, Pd2+, Pt2+, and Au3+ complexes with a d8 configuration tend to form square planar structures because eight electrons occupy the lower orbitals leaving the highest dx2-y2 orbital empty.
Tetrahedral complexes
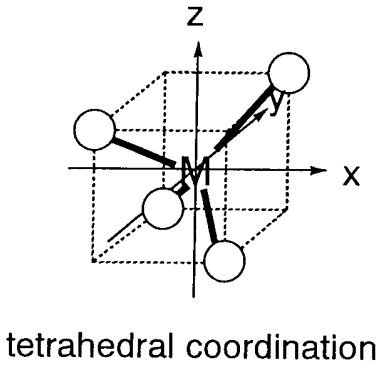
Tetrahedral complexes have four ligands on the apexes of a tetrahedron around the central metal. [CoX4]2- (X = Cl, Br, I), Ni(CO)4, etc. are all examples of 4-coordination complexes (Figure \(\PageIndex{5}\)). When a metal is placed on the origin of the Cartesian axes, as in the octahedral complexes, e orbitals (dx2-y2, dz2) are distant from ligands and t2 orbitals (dxy, dyz, dxz) are nearer ligands. Consequently, the electronic repulsion is larger for the t2 orbitals, which are destabilized relative to the e orbitals. The ligand field exerted by four ligands splits the fivefold degenerate orbitals of the central metal into twofold degenerate e and threefold degenerate t2 sets (Figure \(\PageIndex{6}\)). The t2 set has energy of +2/5 \(\Delta_{t}\) and the e set -3/5 \(\Delta_{t}\) with a ligand field splitting of \(\Delta_{t}\). As the number of the ligands is 4/6 = 2/3 of that in hexacoordinate octahedral complexes, and overlap of the ligands with the orbitals is smaller, and the ligand splitting \(\Delta_{t}\) is about a half of \(\Delta_{o}\). Consequently, only high-spin electron configurations are known in tetrahedral complexes. The ligand field splitting energies calculated by the above method are shown in Table \(\PageIndex{2}\).
| Octahedral | Tetrahedral | ||||||
|---|---|---|---|---|---|---|---|
| Strong field (LS) | Weak field (HS) | ||||||
| dn | Example | n | \(\Delta_{o}\) | n | \(\Delta_{o}\) | n | \(\Delta_{t}\) |
| d1 | Ti3+ | 1 | 0.4 | 1 | 0.4 | 1 | 0.6 |
| d2 | V3+ | 2 | 0.8 | 2 | 0.8 | 2 | 1.2 |
| d3 | Cr3+,V2+ | 3 | 1.2 | 3 | 1.2 | 3 | 0.8 |
| d4 | Cr2+, Mn3+ | 2 | 1.6 | 4 | 0.6 | 4 | 0.4 |
| d5 | Mn2+, Fe3+ | 1 | 2.0 | 5 | 0 | 5 | 0 |
| d6 | Fe2+, Co3+ | 0 | 2.4 | 4 | 0.4 | 4 | 0.6 |
| d7 | Co2+ | 1 | 1.8 | 3 | 0.8 | 3 | 1.2 |
| d8 | Ni2+ | 2 | 1.2 | 2 | 1.2 | 2 | 0.8 |
| d9 | Cu2+ | 1 | 0.6 | 1 | 0.6 | 1 | 0.4 |
| d10 | Cu1+ | 0 | 0 | 0 | 0 | 0 | 0 |
Jahn-Teller Effect
When orbitals of a highly symmetrical nonlinear polyatomic molecule are degenerate, the degeneracy is resolved by distorting the molecular framework to attain lower symmetry and thus lower energy. This is the Jahn-Teller effect and a typical example is seen in the tetragonal distortion of an octahedral coordination structure of hexacoordinate Cu2+ complexes.
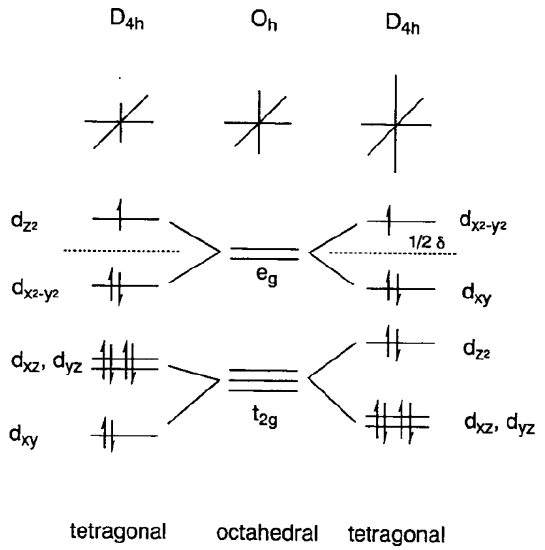
They have a d9 configurations and the eg orbitals in the octahedral structure are occupied by three electrons. If the eg orbitals split and two electrons occupy the lower orbital and one electron the upper orbital, the system gains energy of a half of the energy difference, \(\delta\), of two split orbitals. Therefore a tetragonal distortion in the z axis becomes favorable.

