6.1: Structures of Metal Complexes
- Page ID
- 125406
Transition elements are metallic elements that have incomplete d or f shells in the neutral or cationic states. They are called also transition metals and make up 56 of the 103 elements. These transition metals are classified into the d-block metals, which consist of 3d elements from Sc to Cu, 4d elements from Y to Ag, and 5d elements from Hf to Au, and f-block metals, which consist of lanthanoid elements from La to Lu and actinoid elements from Ac to Lr. Although Sc and Y belong to the d-block, their properties are similar to those of lanthanoids. The chemistry of d-block and f-block elements differs considerably. This chapter describes the properties and chemistry of mainly d-block transition metals.
Central Metals
Properties of d-block transition metals differ considerably between the first (3d) and the second series metals (4d), although the differences in properties between the second and the third series (5d) metals is not pronounced. Metallic radii of elements from scandium, Sc, to copper, Cu, (166 to 128 pm) are significantly smaller than those of yttrium, Y, to silver, Ag, (178 to 144 pm) or those of lanthanum, La, to gold, Au, (188 to 146 pm). Further, metal compounds of the first series transition metals are rarely 7 coordinate, whereas transition metals from the second and third series may be 7 to 9 coordinate. Cerium, Ce, (radius 182 pm) ~ lutetium, Lu, (radius 175 pm) fall between La and Hf and, because of the lanthanide contraction, metallic radii of the second and third series transition metals show little variation.
Higher oxidation states in the second and third series transition metals are considerably more stable than those in the first series transition metals. Examples include tungsten hexachloride, WCl6, osmium tetroxide, OsO4, and platinum hexafluoride, PtF6. Compounds of the first series transition metals in higher oxidation states are strong oxidants and thus are readily reduced. On the other hand, whereas M(II) and M(III) compounds are common among the first series transition metals, these oxidation states are generally uncommon in compounds of second and third series metals. For example, there are relatively few Mo(III) or W(III) compounds compared with many Cr(III) ones. Aqua ions (ions with water ligands) are very common among compounds of first series metals but few are known amongst the second and third metal compounds.
Metal carbonyl cluster compounds of first series transition metals with M-M bonds in low oxidation states exist but halide or sulfide cluster compounds are rare. In general, metal-metal bonds are formed much more easily in the 4d and 5d metals than in the 3d ones. Magnetic moments of the first series transition metal compounds can be explained in terms of spin-only values (cf. Chapter 6.2 (d)) but it is difficult to account for the magnetic moments of the second and third series compounds unless complex factors such as spin-orbital interactions are taken into account.
Thus, it is necessary to acknowledge and understand the significant differences in chemical properties that exist between metals of the first and later series metal compounds, even for elements in the same group.
Properties of the d-block transition metals are different not only in the upper and lower positions in the periodic table but also in the left and right groups. The Group 3 to 5 metals are now often referred to as early transition metals and they are generally oxophilic and halophilic. Smaller numbers of d electrons and the hardness of these elements explain their affinity toward hard oxygen and halogens. In the absence of bridging ligands, the formation of metal-metal bonds is difficult for these elements. Organometallic compounds of these metals are known strongly to activate C-H bonds in hydrocarbons. Late transition metals in the groups to the right of the periodic table are soft and have a high affinity toward sulfur or selenium.
The d-block transition metals have s, p, and d orbitals and those with n electrons in the d orbitals are termed ions with a dn configuration. For example, Ti3+ is a d1 ion, and Co3+ a d6 ion. The number of electrons occupying the orbitals split by the ligand field (cf. 6.2(a)) is denoted by a superscript on the orbital symbol. For example, an ion with 3 electrons in t2g and 2 electrons in eg is described as t2g3eg1.
Ligands
Compounds of metal ions coordinated by ligands are referred to as metal complexes. Most ligands are neutral or anionic substances but cationic ones, such as the tropylium cation, are also known. Neutral ligands, such as ammonia, NH3, or carbon monoxide, CO, are independently stable molecules in their free states, whereas anionic ligands, such as Cl- or C5H5-, are stabilized only when they are coordinated to central metals. Representative ligands are listed in Table \(\PageIndex{1}\) according to the ligating elements. Common ligands or those with complicated chemical formula are expressed in abbreviated forms.
Those ligands with a single ligating atom are called monodentate ligands, and those with more than one ligating atoms referred to as polydentate ligands, which are also called chelate ligands. The number of atoms bonded to a central metal is the coordination number.
| Name | Abbreviation | Formula |
|---|---|---|
| hydrido | H- | |
| carbonyl | CO | |
| cyano | CN- | |
| methyl | Me | CH3- |
| cyclopentadienyl | Cp | C5H5- |
| carbonato | CO32- | |
| ammine | NH3 | |
| pyridine | py | C5H5N |
| bipyridine | bipy | C10H8N2 |
| triphenylphosphine | PPh3 | P(C6H5)3 |
| aqua | aq | H2O |
| acetylacetonato | acac | CH3C(O)CH2C(O)CH3- |
| thiocyanato | SCN- | |
| chloro | Cl- | |
| ethylenediaminetetraacetato | edta | (OOCCH2)2NCH2CH2N(CH2COO)24- |
Coordination number and structures
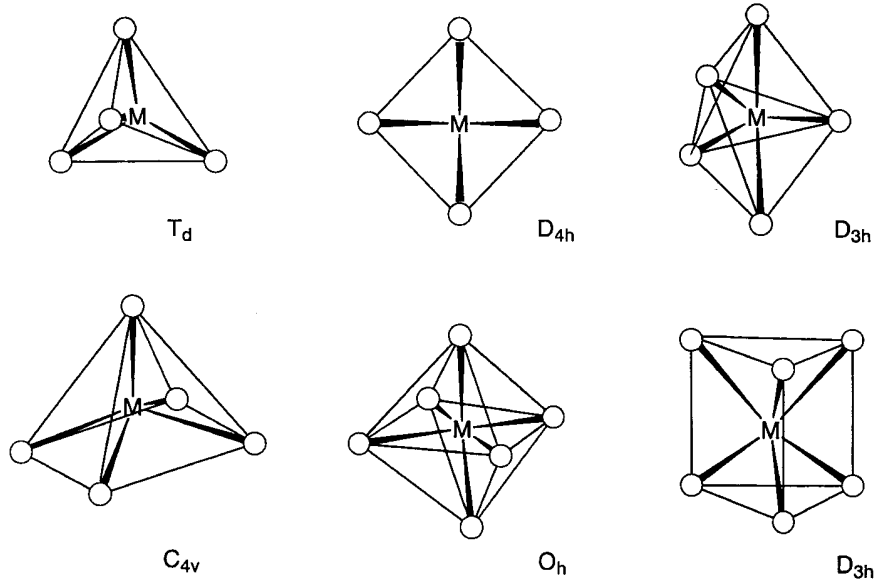
Molecular compounds which consist of d-block transition metals and ligands are referred to as complexes or coordination compounds. The coordination number is determined by the size of the central metal, the number of d electrons, or steric effects arising from the ligands. Complexes with coordination numbers between 2 and 9 are known. In particular 4 to 6 coordination are the most stable electronically and geometrically and complexes with these coordination numbers are the most numerous (Figure \(\PageIndex{1}\)). Complexes with the respective coordination numbers are described below.
Two co-ordinate complexes
Many electron-rich d10 ions, viz: Cu+, Ag+, and Au+, form linear complexes such as [Cl-Ag-Cl]- or [H3N-Au-NH3]-. A zero-valent complex [Pd(PCy3)2] with very bulky tricyclohexylphosphine ligands is also known. Generally, stable 2-coordinate complexes are known for the late transition metals.
Three co-ordinate complexes
Although [Fe{N(SiMe3)3}3] is one example, very few 3-coordinate complexes are known.
Four co-ordinate complexes
When four ligands coordinate to a metal, tetrahedral (Td) coordination is the least congested geometry, although a number of square planar (D4h) complexes are known. [CoBr4]2-, Ni(CO)4, [Cu(py)4]+, [AuCl4]- are all examples of tetrahedral complexes. There are a few known examples of square planar complexes with identical ligands, such as [Ni(CN)4]2-, or [PdCl4]2-. In the case of mixed ligand complexes, a number of square planar complexes of d8 ions, Rh+, Ir+, Pd2+, Pt2+, and Au3+, have been reported. Examples include [RhCl(PMe3)3], [IrCl(CO)(PMe3)2], [NiCl2(PEt3)2], and [PtCl2(NH3)2] (Et = C2H5).
Cis and trans geometrical isomers are possible for complexes with two different kinds of ligands, and were first noted when A. Werner synthesized 4-coordinate [PtCl2(NH3)2]. As tetrahedral complexes do not give geometrical isomers, Werner was able to conclude that his 4-coordinate complexes were square planar. Recently cis-[PtCl2(NH3)2] (Cisplatin) has been used for the treatment of tumors and it is noteworthy that only the cis isomer is active.
Exercise \(\PageIndex{1}\)
Write the formal name of cis-[PtCl2(NH3)2].
- Answer
-
cis-diamminedichloroplatinum
Five co-ordinate complexes
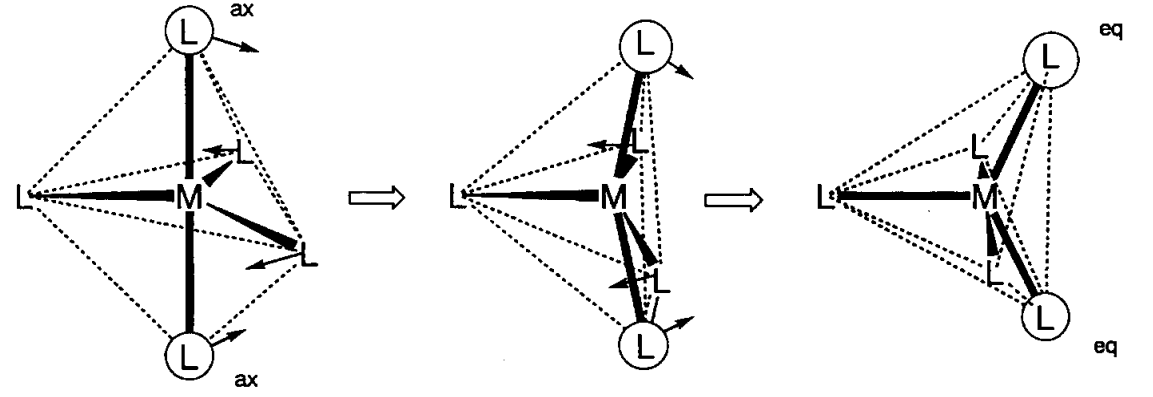
Trigonal bipyramidal (D3h) Fe(CO)5 or square pyramid (C4v) VO(OH2)4 are examples of 5-coordinate complexes. Previously, 5-coordinate complexes were rare but the number of new complexes with this coordination is increasing. The energy difference between the two coordination modes is not large and structural transformation readily occurs. For example, the molecular structure and infrared spectrum of Fe(CO)5 are consistent with a trigonal bipyramid structure, but the 13C NMR spectrum shows only one signal at the possible lowest temperature, which indicates that the axial and equatorial carbonyl ligands are fluxional in the NMR time scale (10-1~10-9 s). Structural transformation takes place via a square pyramid structure and the mechanism is well known as Berry’s pseudorotation.
Six coordinate complexes
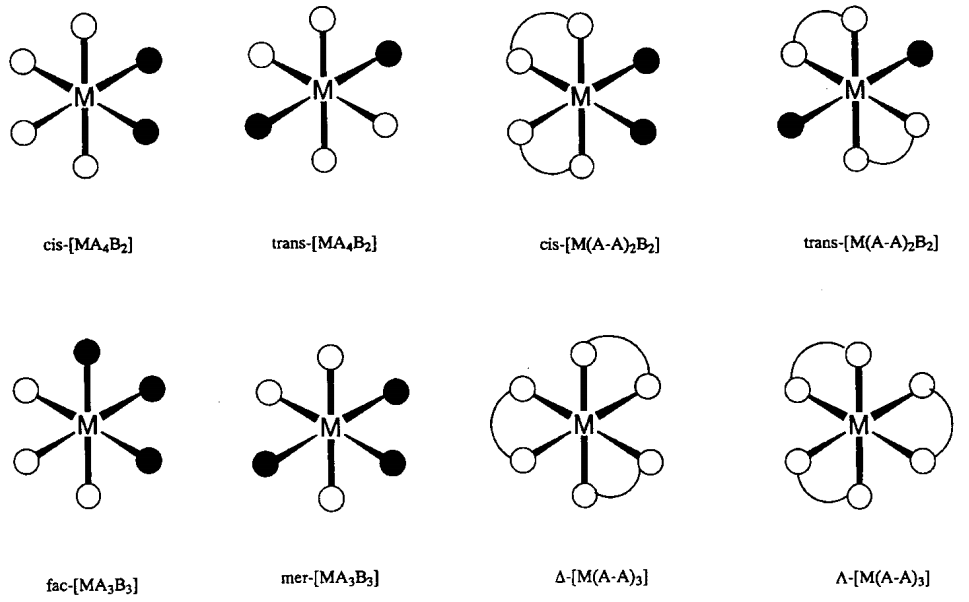
When six ligands coordinate to a central metal, octahedral (Oh) coordination is the most stable geometry and the majority of such complexes assume this structure. In particular, there are a number of Cr3+ and Co3+ complexes which are inert to ligand exchange reactions, represented by [Cr(NH3)6]3+ or [Co(NH3)6]3+. They have been particularly important in the history of the development of coordination chemistry. [Mo(CO)6], [RhCl6]3-, etc. are also octahedral complexes. In the case of mixed ligands, cis- and trans-[MA4B2] and mer- and fac-[MA3B3] geometrical isomers, and for chelate ligands, \(\Delta\)-[M(A-A)3] and \(\Lambda\)-[M(A-A)3] optical isomers (Figure \(\PageIndex{3}\)) are possible. The octahedral structure shows tetragonal (D4h), rhombic (D2h), or trigonal (D3h) distortions caused by electronic or steric effects. The tetragonal distortion of [Cu(NH3)6]2+ by an electronic factor is a typical example of the Jahn-Teller effect (refer to 6.2(a)).
Six ligating atoms can assume trigonal prism coordination. Although this coordination is seen in [Zr(CH3)6]2- or [Re{S2C2(CF3)2}3], few metal complexes with this coordination structure are known because octahedral coordination is sterically less strained. This notwithstanding, it has long been known that the bonding mode of sulfur atoms around a metal is trigonal prism in solid-state MoS2 and WS2.
Exercise \(\PageIndex{2}\)
Write the chemical formula of potassium diamminetetra(isothiocyanato)chromate(III).
- Answer
-
K[Cr(NCS)4(NH3)2]
Higher co-ordinate complexes
Metal ions of the second and third transition metal series can sometimes bond with more than seven ligating atoms and examples are [Mo(CN)8]3- or [ReH9]2-. In these cases, smaller ligands are favorable to reduce steric congestion.

