5.4: Group 13 Metals
- Page ID
- 125402
Aluminum, Al, among Group 13 metals (Table \(\PageIndex{4}\)) exists as aluminosilicates in the Earth’s crust and is more abundant than iron. The most important mineral for metallurgy is bauxite, AlOx(OH)3-2x (0 < x < 1). Although the Al metal was as valuable as noble metals in the 19th century, the price fell dramatically after it came to be manufactured in large quantities by electrolysis of alumina, Al2O3, melted in cryolite, Na3AlF6. However, because its production requires consumption of a large amount of electrical power, the metallurgy of aluminum is economically feasible only in countries where the price of electrical power is low. Therefore, Japan has withdrawn from aluminum smelting, but Japan’s consumption of the metal is second only to the United States. The properties of aluminum are well known as it is widely used and encountered in every day life, for example in one-yen coins, aluminum foil, cooking pans, aluminum window sashes, etc. Aluminum metal usually exceeds 99% purity, and the metal itself and its alloys, like duralumin, are widely used.
| mp (°C) |
bp (°C) |
d(20 °C) (g cm-3) |
E0 (V) M3++3e- |
I first |
(kJ mol-1) second |
third |
|
|---|---|---|---|---|---|---|---|
| Al | 660 | 2519 | 2.70 | -1.66 | 577 | 1816 | 2744 |
| Ga | 29.8 | 2204 | 5.90 | -0.55 | 579 | 1979 | 2962 |
| In | 157 | 2072 | 7.31 | -0.34 | 558 | 1820 | 2704 |
| Tl | 304 | 1473 | 11.9 | +0.74 | 589 | 1971 | 2877 |
Aluminum metal dissolves in mineral acids, except concentrated nitric acid, and in aqueous solutions of alkali metal hydroxides evolving hydrogen. Aluminum forms compounds with most nonmetallic elements and shows a rich chemistry, but unlike boron, no cluster hydrides are known. As oxide and halides have already been described (4.3 (c), 4.5 (d)), organo-aluminum compounds will be mentioned here.
Organoaluminum compounds
Organoaluminum compounds are used in large quantities for olefin polymerization, and they are industrially manufactured from aluminum metal, hydrogen, and an olefin as follows.
\[\ce{2 Al + 3 H2 + 6 CH2 = CHR \rightarrow Al_{2} (CH2CH2R)6}\]
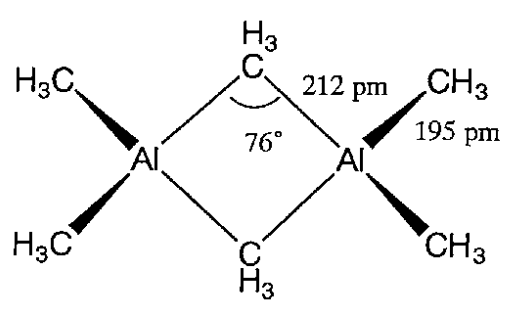
They are dimers except those with bulky hydrocarbyl groups. For example, trimethylaluminum, Al2(CH3)6, is a dimer in which methyl groups bridge aluminum atoms by electron deficient bonds (Figure \(\PageIndex{2}\)).
Organnoaluminum compounds are very reactive and burn spontaneously in air. They react violently with water and form saturated hydrocarbons, with aluminium changing to aluminium hydroxide as follows.
\[\ce{Al(CH2CH3)3 + 3 H2O \rightarrow 3 C2H6 9 Al(OH)3}\]
Therefore, they should be handled in the laboratory under a perfectly inert atmosphere. The Ziegler-Natta catalyst, comprising an organoaluminium compound and a transition metal compound was an epoch-making olefin polymerization catalyst developed in the 1950s, for which the Nobel prize (1963) was awarded.
A transition-metal alkyl compound is formed when an organoaluminum compound reacts with a transition-metal compound. The transition-metal alkyl compound so formed can be isolated when stabilization ligands are coordinated to the metal center.
Gallium, Ga, has the largest temperature difference of the melting point and boiling point among all the metals. Since it melts slightly above a room temperature, the temperature range of the liquid state is very wide and it is used as a high temperature thermometer. In recent years, the metal is used for the manufacture of the compound semiconductors gallium arsenide, GaAs, and gallium phosphide, GaP.
Indium, In, is a soft metal also with a low melting-point. It is the raw material for compound semiconductors InP, InAs, etc. Indium has two stable states, In (I) or In (III), and In (II) compounds are considered to be mixed-valence compounds of monovalent and trivalent In.
Thallium, Tl, also has two stable states, Tl (I) and Tl (III), and Tl (II) is a mixed valence compound of monovalent and trivalent Tl. Since the element is very poisonous the metal and its compounds should be handled carefully. As it is a weak reductant compared to Na(C5H5), thallium cyclopentadienide, Tl(C5H5), is sometimes used for the preparation of cyclopentadienyl compounds, and is a useful reagent in organometallic chemistry.
Exercise \(\PageIndex{3}\)
Give the example of the metals for which stable ions differing in oxidation numbers exist.
- Answer
-
In(I), In(III), Tl(I), Tl(III), Sn(II), Sn(IV).
The reaction of organoaluminum compounds
Organoaluminum compounds were synthesized for the first time in 1859, but they were not regarded as important as Grignard reagents or organolithium compounds as synthetic reagents for some time. This is in part due to the low reactivity of the ether adducts, R3Al:OEt2, which were present because of the frequent use of ether as a solvent. The studies of K. Ziegler changed this situation. K. Ziegler also discovered oligomerization of ethylene by organoaluminum compounds and the formation of higher organoaluminum compounds by the insertion of ethylene in aluminum-carbon bonds. Since alcohols were formed by hydrolysis of organoaluminum compounds, these discoveries were important for organic synthesis.
The discovery of the action of trace amount of nickel in the reaction vessel to give only butene from ethylene led to investigation of the effect of transition metals upon this reaction. Many transition metal salts were examined and Ziegler discovered that titanium compounds gave the highest degree of polymerization of ethylene. This was the birth of the so-called Ziegler catalysts. It should be remembered that this great discovery of the 1950s occurred when the petrochemical industry was beginning to develop and revolutionized the chemical industry of higher polymers.

