1.3: Electronic Structure of Elements
- Page ID
- 125373
Wave functions of electrons in an atom are called atomic orbitals. An atomic orbital is expressed using three quantum numbers; the principal quantum number, n; the azimuthal quantum number, l; and the magnetic quantum number, m\(ell\). For a principal quantum number n, there are n azimuthal quantum numbers l ranging from 0 to n-1, and each corresponds to the following orbitals.
\[\begin{split} \ell & : 0, 1, 2, 3, 4, \ldots \\ & \; \; \; s, p, d, f, g, \ldots \end{split}\]
An atomic orbital is expressed by the combination of n and l. For example, n is 3 and l is 2 for a 3d orbital. There are 2l+1 m\(ell\) values, namely l, l-1, l-2,..., -l. Consequently there are one s orbital, three p orbitals, five d orbitals and seven f orbitals. The three aforementioned quantum numbers are used to express the distribution of the electrons in hydrogen-type atom, and another quantum number ms (1/2, -1/2) which describes the direction of an electron spin is necessary to completely describe an electronic state. Therefore, an electronic state is defined by four quantum numbers (n, l, m\(ell\), ms).
The wave function \(\psi\) which determines the orbital shape can be expressed as the product of a radial wavefunction R and an angular wave function Y as follows.
\[\psi_{n, l, m_{l}} = R_{n, l} (r) Y_{l, m_{l}} (\theta, \phi)\]
R is a function of distance from the nucleus, and Y expresses the angular component of the orbital. Orbital shapes are shown in Figure \(\PageIndex{1}\). Since the probability of the electron’s existence is proportional to the square of the wave function, an electron density map resembles that of a wave function. The following conditions must be satisfied when each orbital is filled with electrons.
[The conditions of electron filling]
Pauli principle
The number of electrons that are allowed to occupy an orbital must be limited to one or two, and, for the latter case, their spins must be anti-parallel (different direction).
Hund's rule
When there are equal-energy orbitals, electrons occupy separate orbitals nd their spins are parallel (same direction).
The order of orbital energy of a neutral atom is
\[1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p \ldots\]
and the electron configuration is determined as electrons occupy orbitals in this order according to the Pauli principle and Hund's rule. An s orbital with one m\(ell\) can accommodate 2 electrons, a p orbital with three m\(ell\) 6 electrons, and a d orbital with five m\(ell\) 0 electrons.
Exercise \(\PageIndex{1}\)
Describe the electron configuration of a C atom, an Fe atom, and a Au atom.
- Answer
-
Electrons equal to the atomic number are arranged in the order of orbital energies. Since the electrons inside the valence shell take the rare gas configuration, they may be denoted by the symbol of a rare gas element in brackets.
\[\begin{split} C & : 1s^{2} 2s^{2} 2p^{2} \quad or \quad [He] 2s^{2} 2p^{2} \\ Fe & : 1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 3d^{6} 4s^{2} \quad or \quad [Ar] 3d^{6} 4s^{2} \\ Au & : 1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 3d^{10} 4s^{2} 4p^{6} 4d^{10} 4f^{14} 5s^{2} 5p^{6} 5d^{10} 6s^{1} \quad or \quad [Xe] 4f^{14} 5d^{10} 6s^{1} \end{split}\]
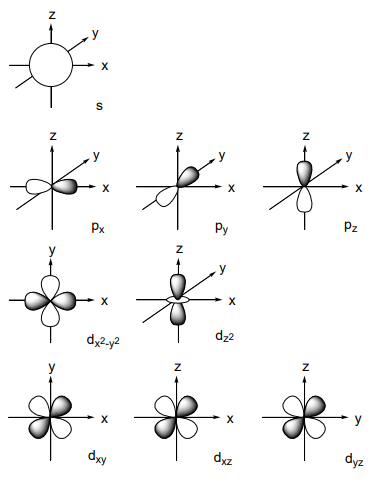
Figure \(\PageIndex{1}\): - Shapes of s, p, and d orbitals. Table \(\PageIndex{1}\) Periodic table of elements. The values are atomic weights 1 2 3 4 5 6 7 8 9 1 1.008
1H2 6.941
3Li9.012
4Be3 22.99
11Na24.31
12Mg4 39.10
19K40.08
20Ca44.96
21Sc47.87
22Ti50.94
23V52.00
24Cr54.94
25Mn55.85
26Fe58.93
27Co5 85.47
37Rb87.62
38Sr88.91
39Y91.22
40Z92.91
41Nb95.94
42Mo(99)
43Tc101.1
44Ru102.9
45Rh6 132.9
55Cs137.3
56BaLanthanoid 178.5
72Hf180.9
73Ta183.8
74W186.2
75Re190.2
76Os192.2
77Ir7 (223)
87Fr(226)
88RaActinoid Lanthan-oid 138.9
57La140.1
58Ce140.9
59Pr144.2
60Nd(145)
61Pm150.4
62Sm152.0
63EuActinoid (227)
89Ac232.0
90Th231.0
91Pa238.0
92U(237)
93Np(239)
94Pu(243)
95Am10 11 12 13 14 15 16 17 18 4.003
2He10.81
5B12.01
6C14.01
7N16.00
8O19.00
9F20.18
10Ne26.98
13Al28.09
14Si30.97
15P32.07
16S35.45
17Cl39.95
18Ar58.69
28Ni63.55
29Cu65.39
30Zn69.72
31Ga72.61
32Ge74.92
33As78.96
34Se79.90
35Br83.80
36Kr106.4
46Pd107.9
47Ag112.4
48Cd114.8
49In118.7
50Sn121.8
51Sb127.6
52Te126.9
53I131.3
54Xe195.1
78Pt197.0
79Au200.3
80Hg204.4
81Tl207.2
82Pb209.0
83Bi(210)
84Po(210)
85At(222)
86Rn157.3
64Gd158.9
65Tb162.5
66Dy164.9
67Ho167.3
68Er168.9
69Tm173.0
70Yb175.0
71Lu(247)
96Cm(247)
97Bk(252)
98Cf(252)
99Es(257)
100Fm(258)
101Md(259)
102No(262)
103Lr

