4.4: Oxygen and oxides (Part 2)
- Page ID
- 125608
Molecular oxides
Ruthenium tetroxide, RuO4,(mp 25 °C and bp 40 °C) and osmium tetroxide, OsO4, (mp 40 °C and bp 130 °C) have low melting and boiling points and their structures are molecular. They are prepared by heating the metal powder in an oxygen atmosphere at about 800 °C. The structures are tetrahedral and they are soluble in organic solvents and also slightly soluble in water. OsO4 is used in organic chemistry especially in the preparation of cis-diols by oxidation of C=C double bonds. For example, cyclohexane diol is prepared from cyclohexene. Since these oxides are very volatile and poisonous, they should be handled very carefully.
1-dimensional chain-like oxide
Mercury oxide, HgO, is a red crystallline compound that is formed when mercury nitrate is heated in air. HgO has an infinite zigzag structure. Chromium trioxide, CrO3, is a red crystalline compound with a low melting point (197 °C) and its structure is composed of CrO4 tetrahedra connected in one dimension. The acidity and oxidizing power of chromium trioxide are very high. It is used as an oxidation reagent in organic chemistry.
Two dimensional stratified oxides
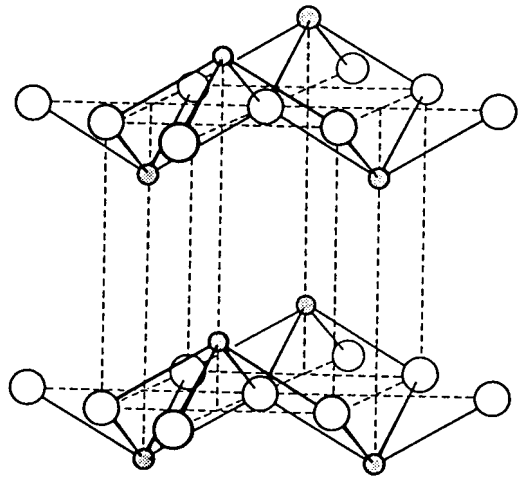
Tetragonal and blue black tin oxide, SnO, and red lead oxide, PbO, are layer compounds composed of square pyramids with the metal atom at the peak and four oxygen atoms at the bottom vertices. The structure contains metal atoms above and below the layer of oxygen atoms alternately and in parallel with the oxygen layers (Figure \(\PageIndex{11}\)). Molybdenum trioxide, MoO3, is formed by burning the metal in oxygen and shows weak oxidizing power in aqueous alkaline solutions. It has a 2-dimensional lamellar structure in which the chains of edge-sharing octahedra MoO6 are corner-linked.
3-dimensional oxides
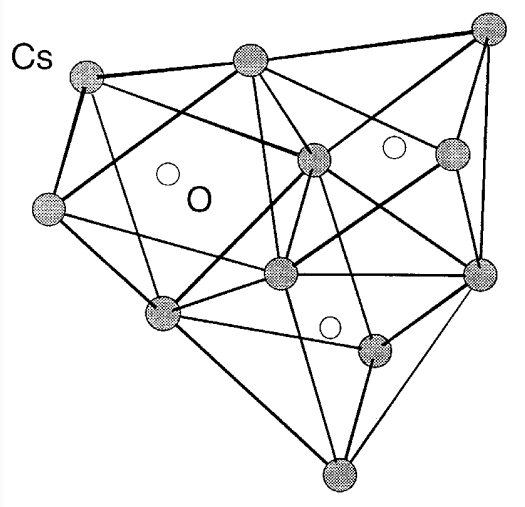
Alkali metal oxides, M2O (M is Li, Na, K, and Rb), have the antifluorite structure (refer to Section 2.2 (e)), and Cs2O is the anti-CdCl2 lamellar structure (refer to Section 4.5 (d)). M2O forms together with peroxide M2O2 when an alkali metal burns in air, but M2O becomes the main product if the amount of oxygen is less than stoichiometric. Alternatively, M2O is obtained by the pyrolysis of M2O2 after complete oxidation of the metal. Peroxide M2O2 (M is Li, Na, K, Rb, and Cs) can be regarded also as the salts of dibasic acid H2O2. Na2O2 is used industrially as a bleaching agent. Superoxide MO2 (M is K, Rb, and Cs) contains paramagnetic ion O2-, and is stabilized by the large alkali metal cation. If there is a deficit of oxygen during the oxidation reactions of alkali metals, suboxides like Rb9O2 or Cs11O3 form. These suboxides exhibit metallic properties and have interesting cluster structures (Figure \(\PageIndex{12}\)). Many other oxides in which the ratio of an akali metal and oxygen varies, such as M2O3, have also been synthesized.
MO type metal oxides
Except for BeO (Wurtz type), the basic structure of Group 2 metal oxides MO is the rock salt structure. They are obtained by calcination of the metal carbonates. Their melting points are very high and all are refractory. Especially quicklime, CaO, is produced and used in large quantities. The basic structure of transition metal oxides MO (M is Ti, Zr, V, Mn, Fe, Co, Ni, Eu, Th, and U) is also the rock salt structure, but they have defect structures and the ratios of a metal and oxygen are non-stoichiometric. For example, FeO has the composition FexO (x = 0.89-0.96) at 1000 °C. The charge imbalance of the charge is compensated by the partial oxidation of Fe2+ into Fe3+. NbO has a defective rock salt-type structure where only three NbO units are contained in a unit cell.
MO2 type metal oxides
The dioxides of Sn, Pb, and other transition metals with small ionic radii take rutile-type structures (Figure \(\PageIndex{13}\)), and the dioxides of lanthanide and actinide metals with large ionic radii take fluorite-type structures.
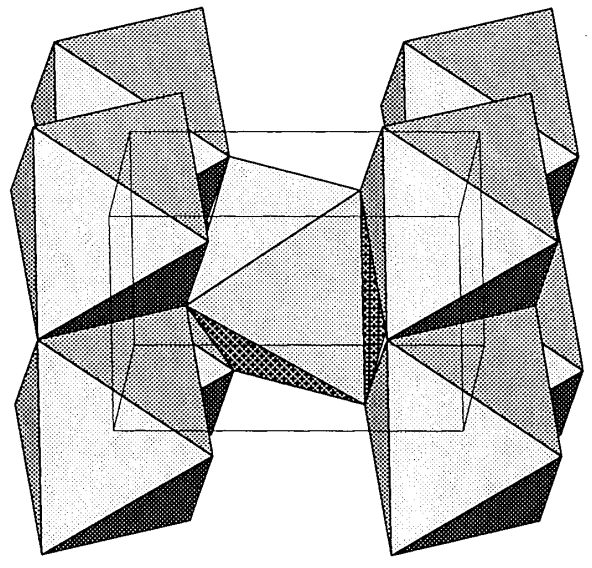
Rutile is one of the three structure types of TiO2, and is the most important compound used in the manufacture of the white pigments. Rutile has also been extensively studied as a water photolysis catalyst. As shown in Figure \(\PageIndex{13}\), the rutile-type structure has TiO6 octahedra connected by the edges and sharing corners. It can be regarded as a deformed hcp array of oxygen atoms in which one half of the octahedral cavities are occupied by titanium atoms. In the normal rutile-type structure, the distance between adjacent M atoms in the edge-sharing octahedra is equal, but some rutile-type metal oxides that exhibit semiconductivity have unequal M-M-M distances. CrO2, RuO2, OsO2, and IrO2 show equal M-M distances and exhibit metallic conductivity.
Manganese dioxide, MnO2, tends to have a non-stoichiometric metal-oxygen ratio when prepared by the reaction of manganese nitrate and air, although the reaction of manganese with oxygen gives almost stoichiometric MnO2 with a rutile structure. The following reaction of manganese dioxide with hydrochloric acid is useful for generating chlorine in a laboratory.
\[MnO_{2} + 4 HCl \rightarrow MnCl_{2} + Cl_{2} + 2 H_{2}O\]
Zirconium dioxide, ZrO2, has a very high melting-point (2700 °C), and is resistant to acids and bases. It is also a hard material and used for crucibles or firebricks. However, since pure zirconium dioxide undergoes phase transitions at 1100 °C and 2300 ºC that result in it breaking up, solid solutions with CaO or MgO are used as fireproof materials. This is called stabilized zirconia.
M2O3-type oxides
The most important structure of the oxides of this composition is the corundum structure (Al, Ga, Ti, V, Cr, Fe, and Rh). In the corundum structure, 2/3 of the octahedral cavities in the hcp array of oxygen atoms are occupied by M3+. Of the two forms of alumina, Al2O3, \(\alpha\) alumina and (\gamma\) alumina, \(\alpha\) alumina takes the corundum structure and is very hard. It is unreactive to water or acids. Alumina is the principal component of jewelry, such as ruby and sapphire. Moreover, various fine ceramics (functional porcelain materials) utilizing the properties of \(\alpha\)-alumina have been developed. On the other hand, \(\gamma\) alumina has a defective spinel-type structure, and it adsorbs water and dissolves in acids, and is the basic component of activated alumina. It has many chemical uses including as a catalyst, a catalyst support, and in chromatography.
MO3 type oxides
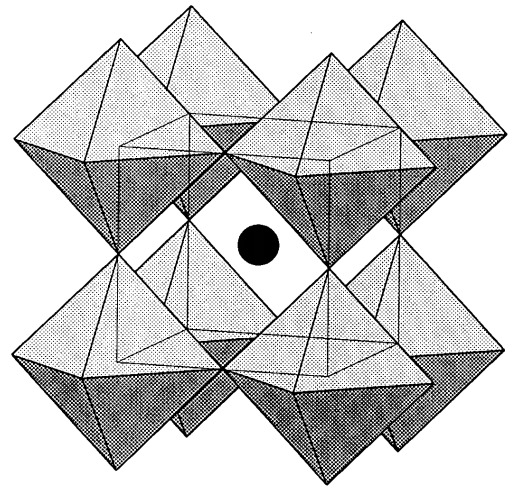
Rhenium and tungsten oxides are important compounds with this composition. Rhenium trioxide, ReO3, is a dark red compound prepared from rhenium and oxygen that has a metallic luster and conductivity. ReO3 has a three-dimensional and very orderly array of ReO6 regular, corner-sharing octahedra (Figure \(\PageIndex{14}\)).
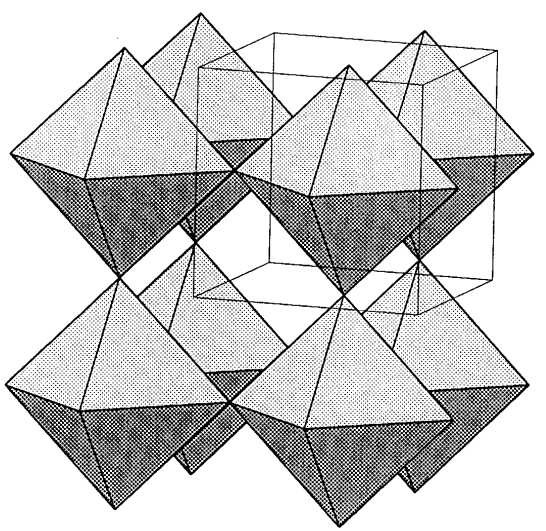
Tungsten trioxide, WO3, is the only oxide that shows various phase transitions near room temperature and at least seven polymorphs are known. These polymorphs have the ReO3-type three-dimensional structure with corner-sharing WO octahedra. When these compounds 6 are heated in a vacuum or with powdered tungsten, reduction takes place and many oxides with complicated compositions (W18O49, W20O58, etc.) are formed. Similar molybdenum oxides are known and they had been regarded as non-stoichiometric compounds before A. Magneli found that they were in fact stoichiometric compounds.
Mixed metal oxides
Spinel, MgAl2O4, has a structure in which Mg2+ occupy 1/8 of the tetrahedral cavities and Al3+ 1/2 of the octahedral cavities of a ccp array of oxygen atoms (Figure \(\PageIndex{15}\)).
Among the oxides of composition A2+B23+O4 (A2+ are Mg, Cr, Mn, Fe, Co, Ni, Cu, Zn, Cd, Sn, and B3+ are Al, Ga, In, Ti, V, Cr, Mn, Fe, Co, Ni, and Rh) , those in which the tetrahedral holes are occupied by A2+ or B3+ are called normal spinels or inverse spinels, respectively. Spinel itself has a normal spinel-type structure, and MgFe2O4 and Fe3O4 have inverse spinel-type structures. Crystal field stabilization energies (refer to Section 6.2 (a)) differ depending on whether the crystal field of the oxygen atoms is a regular trahedron or octahedron. Therefore, when the metal component is a transition metal, the energy difference is one of the factors to determine which of A2+ or B3+ is favorable to fill the tetrahedral cavities.
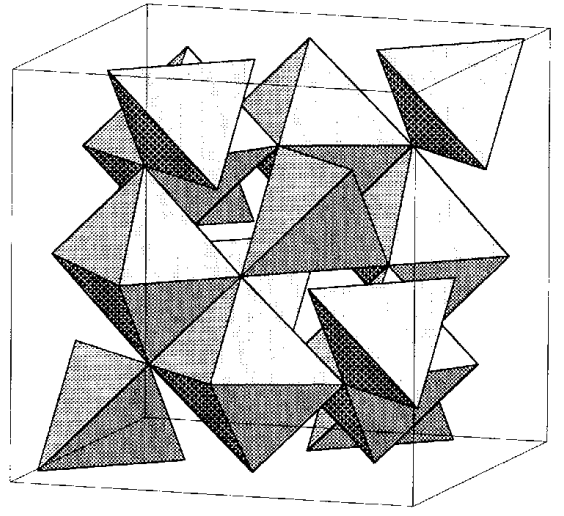
Perovskite, CaTiO3, is an ABO3 oxide (the net charge of A and B becomes 6+), and it has a structure with calcium atom at the center of TiO3 in the ReO3 structure (Figure \(\PageIndex{16}\)). Among this kind of compounds, BaTiO3, commonly called barium titanate, is especially important. This ferroelectric functional material is used in nonlinear resistance devices (varistor).
(h) Oxides of Group 14 elements
Although GeO2 has a rutile-type structure, there is also a \(\beta\) quartz-type polymorphism. There are germanium oxides with various kinds of structures analogous to silicates and aluminosilicates. SnO2 takes a rutile-type structure. SnO2 is used in transparent electrodes, catalysts, and many other applications. Surface treatment with tin oxide enhances heat reflectivity of glasses. PbO2 usually has a rutile-type structure. Lead oxide is strongly oxidizing and used for the manufacture of chemicals, and PbO2 forms in a lead batteries.
(i) Isopolyacids, heteropolyacids, and their salts
There are many polyoxo acids and their salts of Mo(VI) and W (VI). V (V), V (IV), Nb (V), and Ta (V) form similar polyoxo acids although their number is limited. Polyoxoacids are polynuclear anions formed by polymerization of the MO6 coordination polyhedra that share corners or edges. Those consisting only of metal, oxygen, and hydrogen atoms are called isopolyacids and those containing various other elements (P, Si, transition metals, etc.) are called heteropolyacids. The salts of polyacids have counter-cations such as sodium or ammonium instead of protons. The history of polyoxoacids is said to have started with J. Berzelius discovering the first polyoxoacid in 1826, with the formation of yellow precipitates when he acidified an aqueous solution containing Mo (VI) and P (V). The structures of polyoxoacids are now readily analyzed with single crystal X-ray structural analysis, 17O NMR, etc. Because of their usefulness as industrial catalysts or for other purposes, polyoxoacids are again being studied in detail.
Keggin-structure
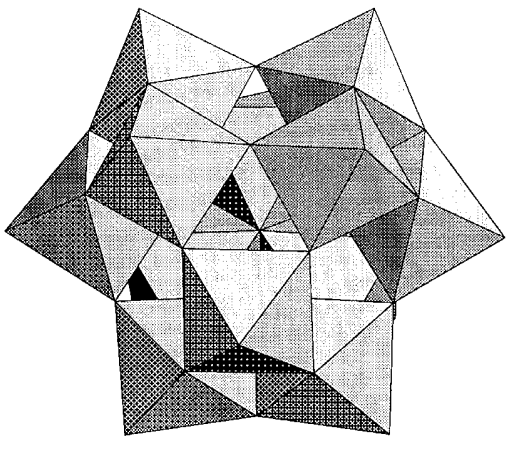
The heteropolyoxo anions expressed with the general formula [Xn+M12O40](8-n)- (M = Mo, W, and X = B, Al, Si, Ge, P, As, Ti, Mn, Fe, Co, Cu, etc.) have the Keggin structure, elucidated by J. F. Keggin in 1934 using X-ray powder diffraction. For example, the structure of the tungstate ion containing silicon, in which 12 WO6 octahedra enclose the central SiO4 tetrahedron and four groups of three edge-shared octahedra connect to each other by corner sharing, is shown in Figure \(\PageIndex{17}\). The four oxygen atoms that coordinate to the silicon atom of the SiO4 tetrahedra also share three WO6 octahedra. Therefore, the whole structure shows Td symmetry. Although the Keggin structure is somewhat complicated, it is very symmetrical and beautiful and is the most typical structure of heteropolyoxo anions. Many other types of heteropolyoxo anions are known.
Polyoxo anions are generated by the condensation of MO6 units by removal of H2O when MoO42- reacts with a proton H+, as is shown in the following equation.
\[12 [MoO_{4}]^{2-} + HPO_{4}^{2-} \xrightarrow{H^{+}} [PMo_{12}O_{40}]^{3-} + 12 H_{2}O\]
Therefore, the size and form of heteropolyoxo anions in the crystal precipitation are decided by the choice of acid, concentration, temperature, or the counter cation for crystallization. A number of studies on the solution chemistry of dissolved anions have been performed.
Heteropolyoxo anions display notable oxidizing properties. As heteropolyoxo anions contain metal ions of the highest oxidation number, they are reduced even by very weak reducing agents and show mixed valence. When Keggin-type anions are reduced by one electron, they show a very deep-blue color. It has been proved that the Keggin structure is preserved at this stage and polyoxo anions absorb more electrons and several M(V) sites are generated. Thus, a heteropolyoxo anion can serve as an electron sink for many electrons, and heteropolyoxo anions exhibit photo-redox reactions.
Exercise \(\PageIndex{4}\)
What is the major difference in the structures of a polyacid and a solid acid?
- Answer
-
Although polyacids are molecules with definite molecular weights, the usual solid oxides have an infinite number of metal-oxygen bonds.

