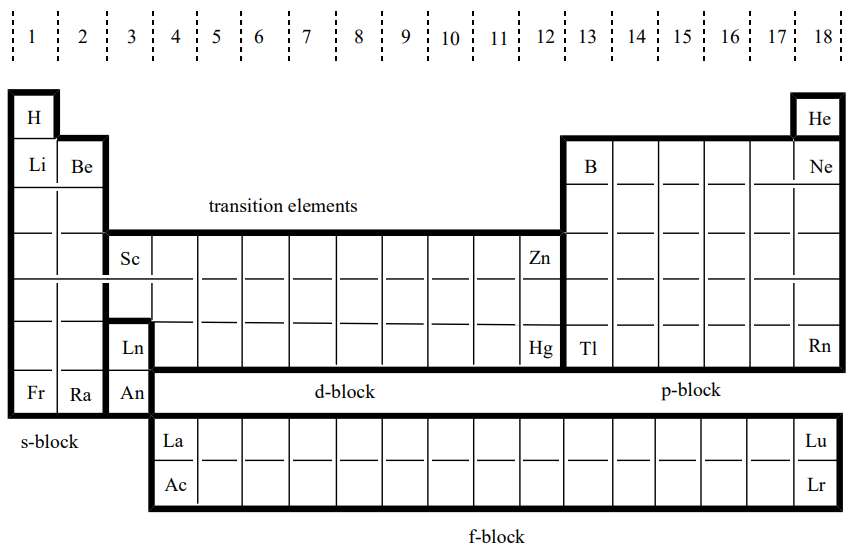
1.4: Block classification of the periodic table and elements
- Page ID
- 125374
Starting from hydrogen, over 100 elements are constituted as electrons are successively accommodated into 1s, 2s, 2p, 3s, 3p, 4s, and 3d orbitals one by one from lower to higher energy levels. When elements with similar properties are arranged in columns, the periodic table of the elements is constructed. The modern periodic table of the elements is based on one published by D. I. Mendeleev in 1892, and a variety of tables have since been devised. The long periodic table recommended by IUPAC is the current standard, and it has the group numbers arranged from Group 1 alkali metals through Group 18 rare gas elements (Table \(\PageIndex{1}\)).
Based on the composition of electron orbitals, hydrogen, helium and Group 1 elements are classified as s-block elements, Group 13 through Group 18 elements p-block elements, Group 3 through Group 12 elements d-block elements, and lanthanoid and actinoid elements f-block elements. (Figure \(\PageIndex{2}\)). s-Block, p-block, and Group 12 elements are called main group elements and d-block elements other than Group 12 and f-block elements are called transition elements. The characteristic properties of the elements that belong to these four blocks are described in Chapter 4 and thereafter. Incidentally, periodic tables that denote the groups of s-block and p-block elements with Roman numerals (I, II,..., VIII) are still used, but they will be unified into the IUPAC system in the near future. Since inorganic chemistry covers the chemistry of all the elements, it is important to understand the features of each element through reference to the periodic table.