8.5: Halides of Phosphorous
- Page ID
- 212662
Phosphorous trihalides
Phosphorous trihalides, PX3, are produced from the direct reaction of phosphorous with the appropriate halogen, (8.5.1). The fluoride is readily made from the halide exchange reaction of PCl3 with fluoride salts, (8.5.2). Mixed trihalides are formed by halide exchange, (8.5.3).
\[ \rm P_4 + 6 Cl_2 \rightarrow 4 PCl_3\]
\[ \rm 2 PCl_3 + 3 CaF_2 \rightarrow 2 PF_3 + 3 CaCl_2\]
\[ \rm PCl_3 + PBr_3 \rightleftharpoons PCl_2Br + PClBr_2\]
A summary of the physical properties of the phosphorous trihalides is given in Table \(\PageIndex{1}\). All the compounds have a pyramidal structure in the vapor phase and in solution.
| Compound | Mp (°C) | Bp (°C) | P-X (Å) | X-P-X (°) |
| PF3 | -151.5 | -101.8 | 1.56 | 96.3 |
| PCl3 | -93.6 | 76.1 | 2.04 | 100 |
| PBr3 | -41.5 | 173.2 | 2.22 | 101 |
| PI3 | 61.2 | 200 (dec.) | 2.43 | 102 |
The phosphorous trihalides hydrolyze to phosphoric acid, (8.5.4), and undergo alcoholysis to form the trialkyl phosphite derivative, (8.5.5). Phosphorous trifluoride is only slowly hydrolyzed by water, but reacts readily with alakaline solutions. In contrast, phosphorus triiodide is an unstable red solid that reacts violently with water. Phosphorous trichloride in particular is an excellent synthon for most trialkyl phosphines, (8.5.6).
\[ \rm PCl_3 + 3 H_2O \rightarrow H_3PO_3 + 3 HCl\]
\[ \rm PCl_3 + 3 HOR \rightarrow P(OR)_3 + 3 HCl\]
\[ \rm PCl_3 + 3 RMgBr \rightarrow PR_3 + 3 MgBrCl\]
As with other P(III) compounds such as trialkyl phosphines, phosphorous trihalides can be oxidized to the analogous phosphene oxide, e.g., (8.5.7).
\[ \rm 2 PCl_3 + O_2 \rightarrow \text{O=P}Cl_3\]
Phosphorous trihalides form Lewis acid-base complexes with main group metals, but the bonding to dn (n ≠ 0) transition metals occurs in the same manner to that of trialkyl phosphine, i.e., with dπ-pπ back donation. In fact one of the first examples of complexation of phosphorous to a low oxidation metal was the formation of PF3 complexes with Fe-porphyrin.
Phosphorous pentahalides
The reaction of white phosphorous with excess halogen yields the pentahalides, (8.5.8). However, the pentafluoride is best prepared by halide exchange, (8.5.9).
\[ \rm P_4 + 10 Cl_2 \rightarrow 4 PCl_5\]
\[ \rm 2 PCl_5 + 5 CaF_2 \rightarrow 2 PF_3 + 5 CaCl_2\]
The pentaiodide is unknown, however, selected physical properties are given for the others in Table \(\PageIndex{2}\).
| Compound | Mp (°C) | Bp (°C) |
| PF5 | -93.78 | -84.5 |
| PCl5 | 166.8 | 160 (subl.) |
| PBr5 | 100 | 106.0 |
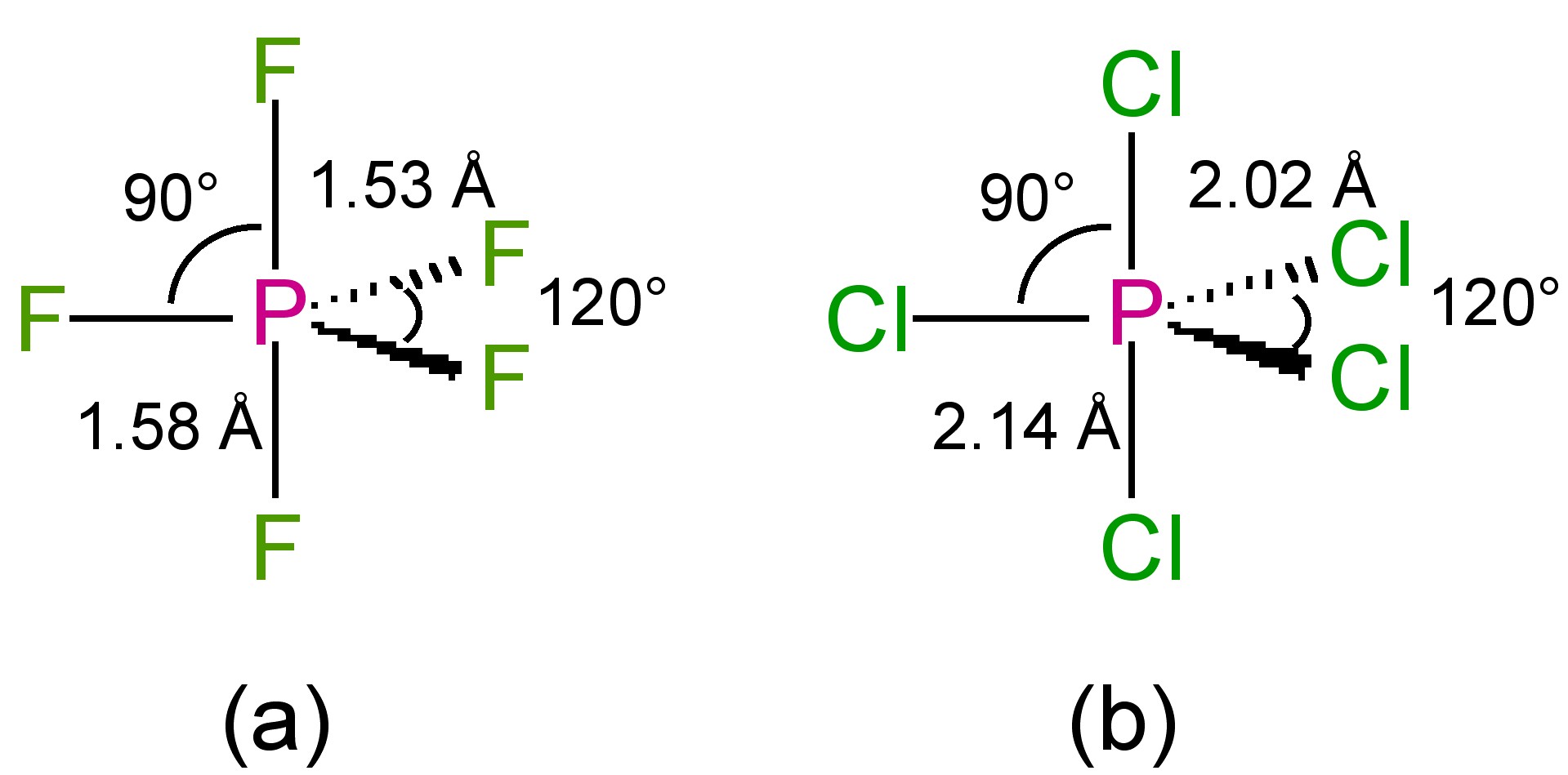
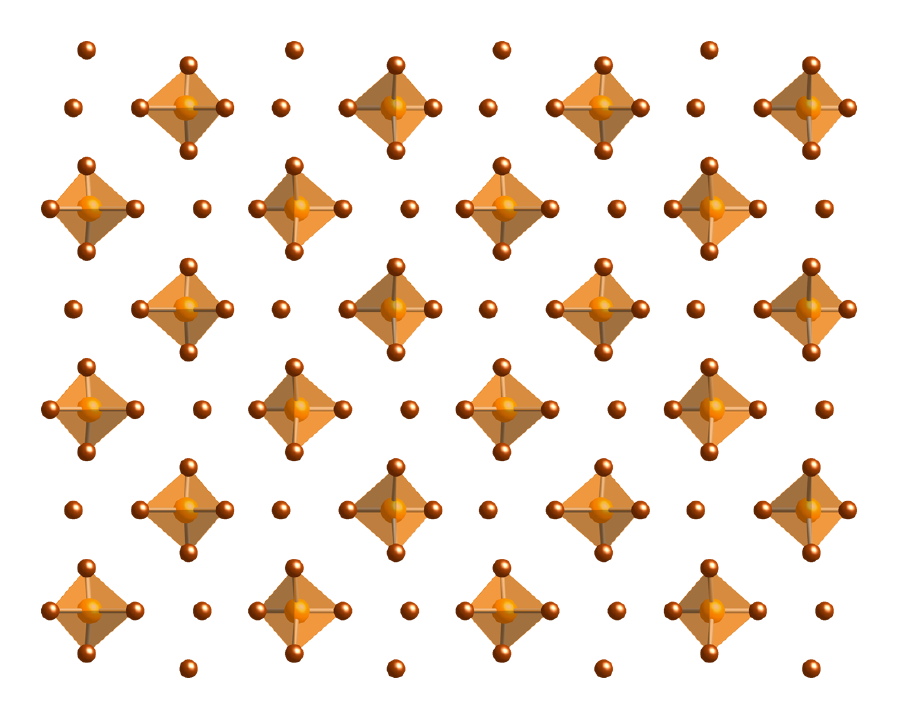
The structures of the phosphorous pentahalides are all trigonal bipyramidal in the gas phase (Figure \(\PageIndex{1}\)). Phosphorous pentafluoride maintains the trigonal bipyramidal structure in solid state, but the chloride and bromide are ionic solids, [PCl4]+[PCl6]- and [PBr4]Br (Figure \(\PageIndex{2}\)), respectively. The tetrahedral [PCl4]+ ion is also formed with the reaction of PCl5 with other chloride ion acceptors, (8.5.10) and (8.5.11).
\[ \rm PCl_5 + TiCl_4 \rightarrow [PCl_4]^+[Ti_2Cl_9]^-\]
\[ \rm PCl_5 + NbCl_5 \rightarrow [PCl_4]^+[NbCl_6]^-\]
All of the pentahalides undergo thermal decomposition, (8.5.12).
\[ \rm PX_5 \rightleftharpoons PX_3 + X_2\]
Careful hydrolysis of the pentahalides yields the oxide of the appropriate trihalide, (8.5.13), while excess hydrolysis yields phosphoric acid, (8.5.14).
\[ \rm PX_5 + H_2O \rightarrow \text{O=}PX_3 + HX\]
\[ \rm PX_5 + 4 H_2O \rightarrow H_3PO_4 + HX\]


