7.5: Carbon Monoxide
- Page ID
- 212655
Carbon monoxide (CO) is iso-electronic with nitrogen (N2) and formed through the incomplete combustion of carbon, (7.5.1), or hydrocarbon compounds.
\[ \text{2 C + O}_2 \rightarrow \text{ 2 CO}\]
Carbon monoxide may also be made from steam and coal as part of synthesis gas, (7.4.2). A convenient laboratory preparation of CO is the dehydration of formic acid by sulfuric acid, (7.4.3).
\[ \text{C + H}_2\text{O} \rightarrow \text{CO + H}_2 \]
\[ \text{HCO}_2\text{H} \rightarrow \text{CO + H}_2\text{O}\]
Hazards and toxicity
Carbon monoxide is flammable, (7.4.4), and has an explosive limit of 12.5 – 74% with an auto ignition temperature of 609 °C.
\[\text{2 CO + O}_2 \rightarrow \text{2 CO}_2\]
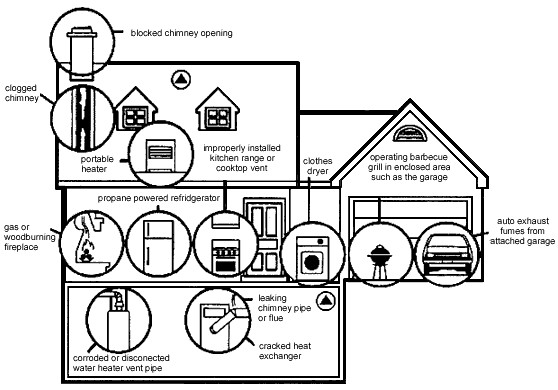
Carbon monoxide is also very toxic; however, it is colorless, odorless, tasteless and non-irritating all of which increase its danger. The incomplete combustion of hydrocarbon (natural gas or heating oil) or carbon sources (coal or charcoal) is a common hazard in the home. In a closed environment (e.g., charcoal grill in room with no, or poor, ventilation) as carbon uses up oxygen in room the formation of toxic carbon monoxide results instead of carbon dioxide (CO2).Typical sources of CO in the home are shown in Figure \(\PageIndex{1}\).
The toxicity of CO is due to its competition with oxygen at the heme binding site in hemoglobin. The binding affinity for CO is 200 times greater than that for oxygen, meaning that just small amounts of CO dramatically reduces hemoglobin's ability to transport oxygen around the body. The bright red color of the CO-heme complex is the reason that chronic exposure results in the skin adopting a bright red color. The symptoms of CO poisoning include headache, nausea, weakness, and eventually death. When air contains CO levels as low as 0.02%, headache and nausea occur; if the CO concentration is increased to 0.1%, unconsciousness will follow.
Note
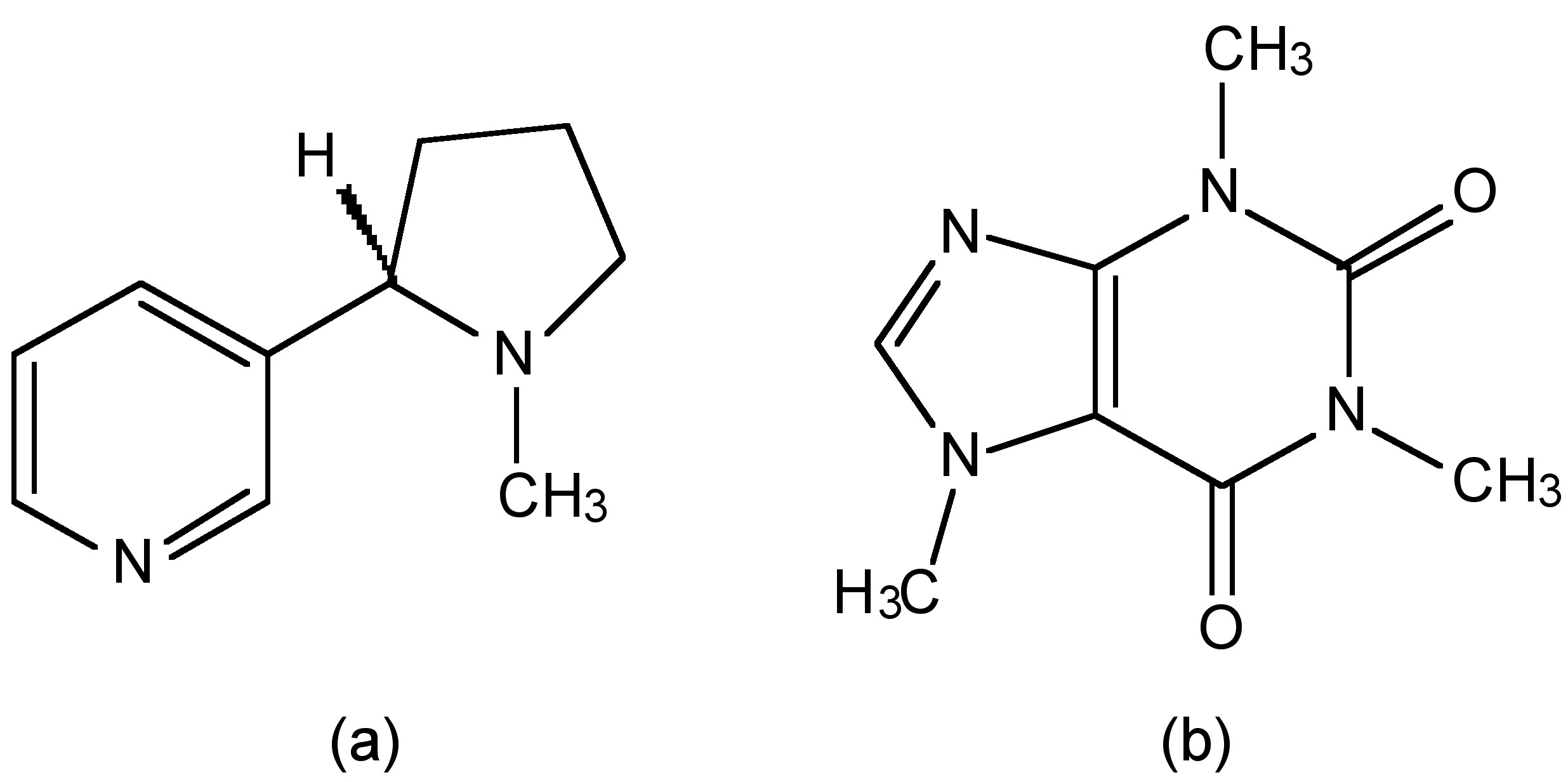
Cigarette smoke containing large amounts of carbon monoxide and as a result heavy smokers can have up to 20% of the oxygen-active sites in their blood blocked by CO. However, despite this hazard, cigarettes would be even more deadly if they were not burnt. A key (and often engineered) ingredient in cigarettes is the addictive drug nicotine. Nicotine is an alkaloid (Figure \(\PageIndex{2}\)a) as is caffeine (Figure \(\PageIndex{2}\)b) that is in coffee and tea. The lethal dose of caffeine is approximately 10 g, which relates to approximately 70 – 100 cups of coffee (assuming a concentration of 100- 150 mg per cup). Alternatively, a lethal dose of caffeine from cola would require approximately 180 – 280 12 oz bottles, each containing 35 – 44 mg. In comparison nicotine has a lethal dose of 50 mg. This means that 12 cigarettes can provide a lethal does if consumed. The only reason smoking 12 cigarettes do not kill immediately is that the majority of the nicotine is burnt in the smoking of the cigarette. If this were not true smokers would be killed before they could develop a habit!
Structure and bonding
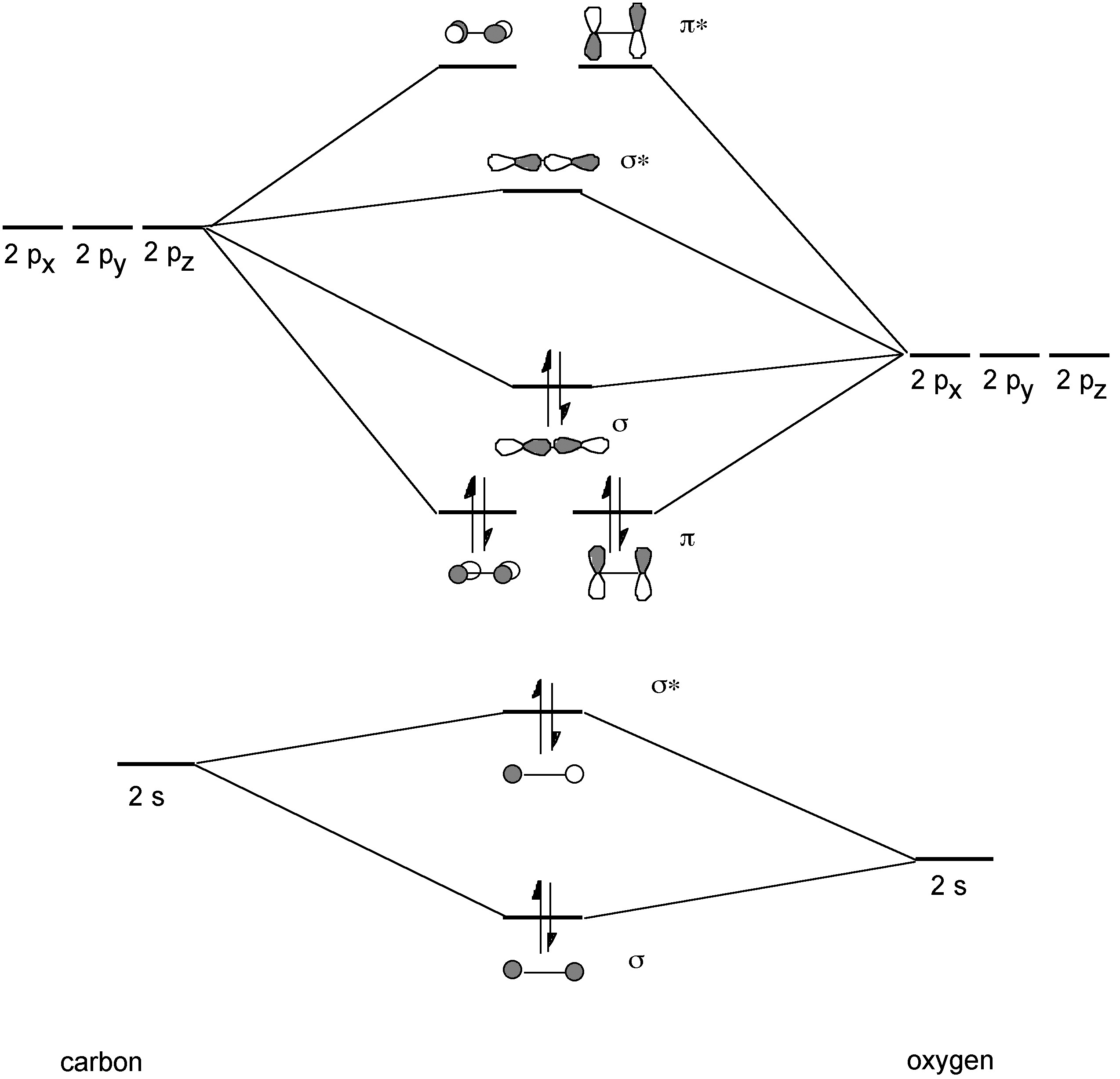
The bonding in CO involves 1 σ-bond and 2 sets of π-bonds (Figure \(\PageIndex{3}\)). The C-O bond distance in carbon monoxide is 1.1 Å and therefore consistent with a triple bond. In comparison a typical C-O single bond is ca. 1.43 Å, and an average C-O double bond is ca. 1.23 Å. The major absorption band in the infra red spectrum for CO is 2143 cm-1, while for 13CO it is 2099.2 cm-1.