3.1: The Alkali Metal Elements
- Page ID
- 212623
The Group 1 metals have a particular name: the alkali metals. This is due to the formation of alkali (basic) solutions upon their reaction with water. Table \(\PageIndex{3}\).1 lists the derivation of the names of the alkali metals.
| Element | Symbol | Name |
|---|---|---|
| Lithium | Li | Greek lithos meaning stone |
| Sodium | Na | Latin natrium or Arabic natrun meaning soda |
| Potassium | K | From the Latin kalium, and from Arabic al-qali meaning plant ashes |
| Rubidium | Rb | Latin rubidus meaning deepest red |
| Caesium | Cs | Latin caesius meaning blueish grey |
| Francium | Fr |
Named after France |
Note
Caesium is the international spelling standardized by the IUPAC, but in the United States it is more commonly spelled as cesium.
Discovery
Lithium
Petalite (Li2O.Al2O3.8SiO2) was first discovered in 1800 by José Bonifácio de Andrade e Silva (Figure \(\PageIndex{3}\).1), who discovered the mineral in a Swedish mine on the island of Utö. However, it was not until 1817 that Johan August Arfwedson (Figure \(\PageIndex{3}\).2) working in the laboratory of Jöns Jakob Berzelius (Figure \(\PageIndex{3}\).3), discovered the presence of a new element while analyzing petalite ore. Named from the Greek lithos meaning stone reflected its discovery in a mineral, as opposed to sodium and potassium, which had been discovered in plant tissue; its name was later standardized as lithium. The element was not isolated until 1821, when William Brande (Figure \(\PageIndex{3}\).4) isolated the element by performing electrolysis on lithium oxide, a process previously employed by Sir Humphry Davy to isolate potassium and sodium.




Sodium
3.1.1.2 Sodium Elemental sodium was first isolated by Sir Humphry Davy (Figure \(\PageIndex{3}\).5) in 1806 by passing an electric current through molten sodium hydroxide.

Potassium
The name kalium was taken from the word alkali, which came from Arabic al qali meaning the calcined ashes. The name potassium was made from the English word potash, meaning an alkali extracted in a pot from the ash of burnt wood or tree leaves. Potassium metal was discovered in 1807 by Sir Humphry Davy (Figure \(\PageIndex{3}\).5), who derived it from caustic potash (KOH), by the use of electrolysis of the molten salt.
Rubidium
Rubidium was discovered using spectroscopy in 1861 by Robert Bunsen (Figure \(\PageIndex{3}\).6) and Gustav Kirchho (Figure \(\PageIndex{3}\).7) in the mineral lepidolite. The first rubidium metal was produced by Bunsen from the reaction of rubidium chloride (RbCl) with potassium.


Caesium
Like Rubidium, caesium was discovered spectroscopically by Bünsen (Figure \(\PageIndex{3}\).6) and Kirchho (Figure \(\PageIndex{3}\).7) in 1860 in mineral water from Drkheim, Germany. The residues of 44,000 liters of mineral water yielded several grams of a caesium salt. Its identification was based upon the bright blue lines in its spectrum and it was the first element discovered by spectral analysis. The first caesium metal was subsequently produced in 1882 by electrolysis of caesium chloride.
Francium
Originally known as eka-caesium, francium was discovered in 1939 by Marguerite Perey (Figure \(\PageIndex{3}\).8) of the Curie Institute in Paris, France when she purified a sample of actinium-227, which had been reported to have a decay energy of 220 keV. However, Perey noticed decay particles with an energy level below 80 keV. Perey thought this decay activity was caused by a previously unidentified element, which exhibited chemical properties of an alkali metal. This led Perey to believe that it was element 87, caused by the alpha decay of actinium-227. Perey named the new isotope actinium-K (now referred to as francium-223) and in 1946, she proposed the name catium for her newly discovered element, however, she subsequently suggested francium, after France.

Abundance
The abundance of the alkali metals is given in Table \(\PageIndex{3}\).2. Sodium's high abundance is due mainly to large underground deposits of rock salt (NaCl). However, sodium is also more abundant in seawater (10,800 ppm) as compared to potassium (380 ppm).
| Element | Terrestrial abundance (ppm) |
|---|---|
| Li | 20 (Earth's crust), 40 (soil), 0.17 (sea water) |
| Na | 23,000 (Earth's crust), 10,500 (sea water) |
| K | 21,000 (Earth's crust), 14 (soil), 380 (sea water) |
| Rb | 90 (Earth's crust), 30 - 250 (soil), 0.1 (sea water) |
| Cs | 3 (Earth's crust), 0.0001 (soil), 0.0003 (sea water) |
| Fr | Essentially nil |
Isotopes
The naturally abundant isotopes of the alkali metals are listed in Table \(\PageIndex{3}\).3. All of the isotopes of francium are radioactive. Lithium-7 and sodium-23 are both useful NMR nucleus having I = 1/2.
| Isotope | Natural abundance (%) |
|---|---|
| Lithium-6 | 7.5 |
| Lithium-7 | 92.5 |
| Sodium-23 | 100 |
| Potassium-39 | 93 |
| Potassium-40 | 0.0118 |
| Potassium-41 | 6.9 |
| Caesium-133 | 100 |
Potassium has three isotopes (Table \(\PageIndex{3}\).3), of which potassium-40 is radioactive and provides the basis for the determination of the age of rocks between 105 and 109 years old, i.e., those formed in proterozoic and cenozoic periods of geological time. The decay of potassium-40 occurs with a half life of 1.31 x 109 years, by two routes. That associated with a beta particle decay accounts for 89% of the decay:
\[${40}_{19}K \rightarrow ^{97}_{20}Ca + ^0_{-1}e\]
While that associated with an electron capture and by positron emission decay accounts for 11% of the decay to give argon-40. Since many rocks contain potassium containing minerals the decay of potassium-40 after solidification of the rock will result in the formation of argon trapped in the rock. The argon-40 content is determined by mass spectrometry, while the potassium content is determined by flame spectrophotometry. The ratio of the two, (3.2), will allow for the determination of the elapsed time since the rock solidified.
\[ \dfrac{[^{40}_{18}Ar]}{[^{40}_{19}K]}\]
Caesium has at least 39 known isotopes (more than any other element except francium) ranging from caesium-112 caesium-151; however, caesium-133 is the only naturally occurring stable isotope. The other isotopes have half-lives from a few days to fractions of a second. The radiogenic isotope caesium-137 is produced from the detonation of nuclear weapons and is produced in nuclear power plants, and was released to the atmosphere most notably from the 1986 Chernobyl accident.
Physical properties
Many of the physical properties of the alkali metals (Table \(\PageIndex{3}\).4) are typical of metals, e.g., thermal and electrical conductivity. However, due to the relatively weak inter-atomic forces (weak M-M bonding) they are soft and readily cut with a knife.
| Element | Melting point (◦C) | Boiling point (◦C) | Density (g/cm3) | Electrical resistivity (Ω·cm) |
|---|---|---|---|---|
| Li | 453 | 1615 | 0.534 | 12.17 @ 86 ◦C |
| Na | 370 | 1156 | 0.968 | 5.23 @ 29 ◦C |
| K | 336 | 1032 | 0.89 | 7.01 @ 22.8 ◦C |
| Rb | 312 | 961 | 1.532 | 12.52 @ 53 ◦C |
| Cs | 201 | 944 | 1.93 | 37.38 @ 28.1 ◦C |
Reactivity
All the alkali metals are highly reactive and are as a consequence of the stability of the M+ ion are strong reducing agents (Table \(\PageIndex{3}\).5). The metals react readily with hydrogen and oxygen.
\[\ce{ M + 1/2 H2 \rightarrow M^{+}H^{-}}\]
\[ \ce{ M + O2 -> M^{+}(O_2)^{-} }\]
| Reduction | Reduction potential (V) |
|---|---|
| Li+ + e- → Li | -3.045 |
| Na+ + e- → Na | -2.7109 |
| K+ + e- → K | -2.924 |
| Rb+ + e- → Rb | -2.925 |
| Cs+ + e- → Cs | -2.923 |
All of the alkali metals react with water to liberate hydrogen.
\[ \ce{ M + H2O -> M(OH) + 1/2H2}\]
WARNING
The reactions of alkali metals with water are exothermic and the heat generated is sucient to ignite the hydrogen. In addition the solutions formed are highly alkaline. Caution should be taken when handling alkali metals and storage should always be under mineral oil.
A similar, but less violent, reaction is also observed with ammonia when catalyzed by transition metal ions.
\[ \ce{M + NH3 -> M(NH2) + 1/2 H2} \]
In the absence of a catalyst, the Group 1 metals dissolve in liquid ammonia to form solutions with characteristic properties.
- Highly reducing.
- Blue color.
- ESR signal due to solvated electrons.
As an example, the dissolution of sodium in liquid ammonia results in the formation of solvated Na+ cations and electrons.
\[ Na_{(s)} \rightarrow Na_{(solv)} \leftrightharpoons Na^+_{(solv)} + e^-_{(solv)} \]
The solvated electrons are stable in liquid ammonia and form a complex: [e-(NH3)6]. It is this solvated electron that gives the strong reducing properties of the solution as well as the characteristic signal in the ESR spectrum associated with a single unpaired electron. The blue color of the solution is often ascribed to these solvated electrons; however, their absorption is in the far infra-red region of the spectrum. A second species, Na-(solv), is actually responsible for the blue color of the solution.
\[ 2 Na_{(solv)} \leftrightharpoons Na^+_{(solv)} + Na^-_{(solv)} \]
The formation of the sodium anion is confirmed by complexation of the cation with a cryptan ligand (C) such as a crown ether.
\[ Na^+_{(solv)} + Na^-_{(solv)} + "C" \rightarrow [Na(C)]^+ + Na^- \]
The resulting complex is found to be isostructural to the iodide analog in the solid state.
\[ Na^+_{(solv)} + I^-_{(solv)} + "C" \rightarrow [Na(C)]^+ + I^- \]
Vapor phase
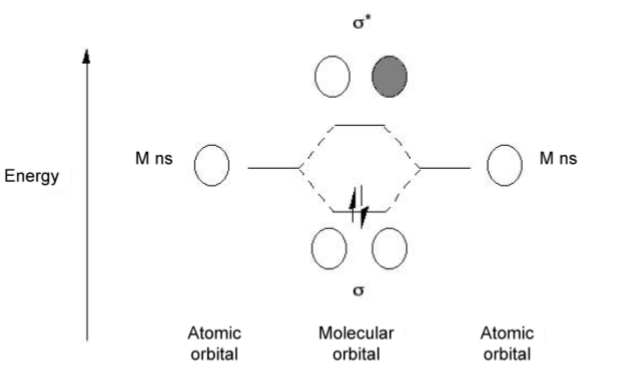
All the alkali metals form M2 dimers in the vapor phase in an analogous manner to hydrogen. As with dihydrogen the bonding is associated with the molecular orbital combination of the two valence s-orbitals (Figure \(\PageIndex{3}\).9).
Sodium vapor is commonly used for lighting in a gas discharge lamp, which uses sodium in an excited state to produce light (Figure \(\PageIndex{3}\).10). There are two varieties of such lamps: low pressure and high pressure.



