9.14: Problems
- Page ID
- 189520
1. Use lattice energies to explain why MgSO4 decomposes to magnesium oxide and SO3 at a much lower temperature than does BaSO4.
2. Solid MgO might be formulated as Mg+O- or Mg2+O2-. Use the thermochemical data below (some of which are irrelevant) and Kapustinskii's formula to determine which is more stable. The lattice constant for MgO (NaCl structure) is 4.213 Å. While the idea of an O- ion might seem strange, note that the second electron affinity of O and the second ionization potential of Mg (in the table below) are both quite endothermic.
| Reaction | ∆Ho, kJ/mol |
|---|---|
| Mg(s) = Mg(g) | 148 |
| Mg(g) = Mg+(g) + e- | 739 |
| Mg+(g) = Mg2+(g) + e- | 1,452 |
| O2(g) = 2 O(g) | 498 |
| O(g) + e- = O-(g) | -141 |
| O-(g) + e- = O2-(g) | 790 |
3. From the heat of formation of solid NH4Cl (-315 kJ/mol) and gaseous NH3 (-46), the bond dissociation energies of H2 (436) and Cl2 (244), the ionization potential of atomic hydrogen (1,311), and the electron affinity of atomic chlorine (-349), calculate the gas-phase proton affinity of NH3. The lattice energy of NH4Cl may be estimated from Kapustinskii's formula using rN-Cl = 3.50 Å.
4. Bottles of aqueous ammonia are often labeled “ammonium hydroxide.” We will test this idea by using a lattice energy calculation to determine whether the salt NH4+OH- can exist.
The heats of formation of gaseous OH- and H2O are respectively -141 and -242 kJ/mol. Assuming that NH4+ is about the same size as Rb+, and OH- about the same size as F-, using Kapustinskii's formula, ionic radii, and the NH3 proton affinity calculated in problem 3, determine whether NH4+OH- should be a stable salt relative to NH3 and H2O. At what temperature should NH4+Cl- be unstable relative to NH3 and HCl, if ΔHfo for HCl is -92 kJ/mol and ΔSo (\(\ce{NH4Cl -> NH3 + HCl}\)) = 280 J/mol K?
5. Lithium metal burns in nitrogen to make the nitride Li3N. The heavier alkali metals (K, Rb, Cs) can form stable azides (MN3), but not M3N nitrides. Explain why this is so.
6. (a) Do you expect BaSO4 or MgSO4 to be more soluble in water? (b) Is LiF more soluble than LiClO4? Explain.
7. Which polymorph of ZnS (zincblende or wurzite) would you expect to be more stable on the basis of electrostatic energy?
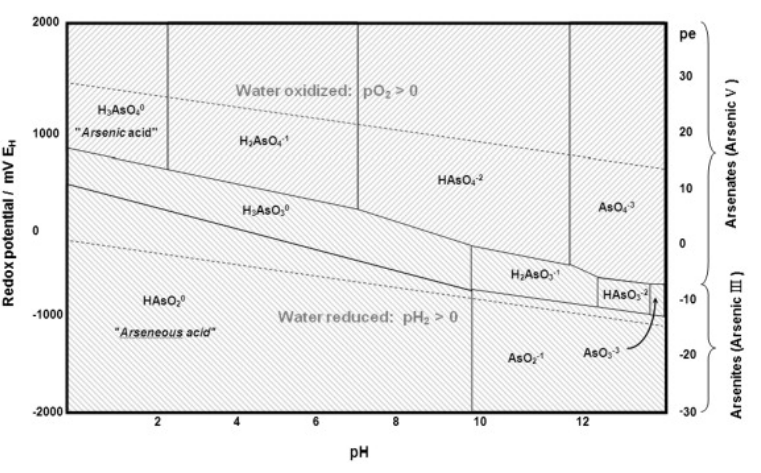
8. Arsenic contamination of ground water is a serious problem in Bangladesh, Chile, Argentina, and other parts of the world including the western United States. Arsenic poisoning been widespread in the Ganges river delta, where tube wells bring contaminated water up from 20-100 meters below the surface. One simple treatment that has been proposed is to precipitate the arsenic by aeration of the well water, which also contains high concentrations of Fe2+. Referring to the Pourbaix diagram of arsenic below and the Pourbaix diagram of iron in Chapter 4, identify the iron and arsenic species that are present in aerated water at neutral pH. What insoluble compound precipitates to lower the concentration of arsenic? (Hint: which compound would have the largest lattice energy?)