9.8: Oxides and Oxyacids of Sulfur
- Page ID
- 212872
Note
Alternative spellings of sulfurous and sulfuric acids are based upon the traditional UK spelling of sulphur, i.e., sulphurous and sulphuric acid.
Sulfur dioxide and sulfurous acid solutions
The combustion of sulfur results in the formation of gaseous sulfur dioxide, (9.8.1).
\[ \rm S + O_2 \rightarrow SO_2\]
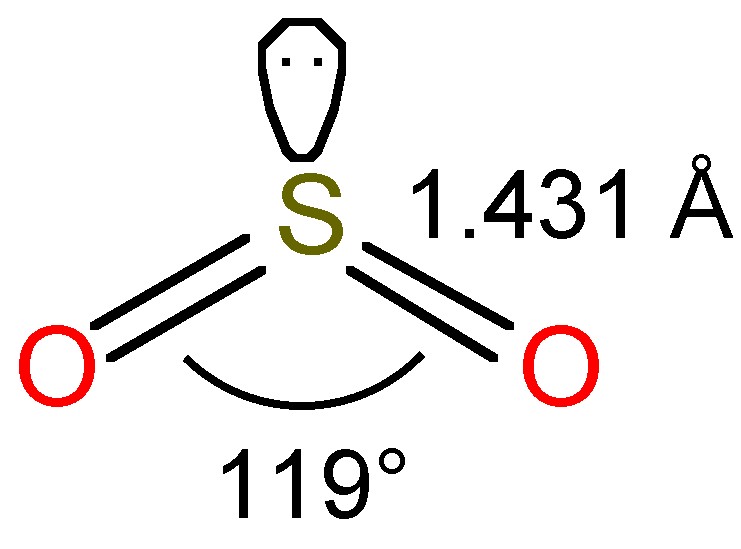
The bent structure of SO2 is shown in Figure \(\PageIndex{1}\), and as a consequence of the sp2 hybridization the molecule is polar.
The modest boiling temperature of SO2 (-10 °C) means that it is readily liquefied and easily kept as a liquid at room temperature under a slight pressure. The liquid is associated by dipole-dipole attractions due to the polar nature of SO2. Liquid SO2 is a good solvent due to the polarity of the molecule; as a consequence it readily solubalizes polar compounds and salts. It is also convenient since it is easy to remove from reaction products by evaporation.
Sulfur dioxide is soluble in water forming aqueous solutions where most of the SO2 is maintained as a hydrogen-bonded hydrate, in a similar manner to that observed for aqueous solutions of carbon dioxide. At equilibrium in neutral water (no added base) a small fraction reacts, to give a mixture of bisulfite (HSO3-, Figure \(\PageIndex{2}\)a) and sulfite (SO32-, Figure \(\PageIndex{2}\)b), (9.8.2). The free acid does not to exist.
\[ \rm SO_{2(aq)} + H_2O \rightleftharpoons \underset{\underset{H-SO_3^-}{\downarrow}}{HOSO_{2(aq)}^-} \rightleftharpoons SO_{3(aq)}^{2-}\]
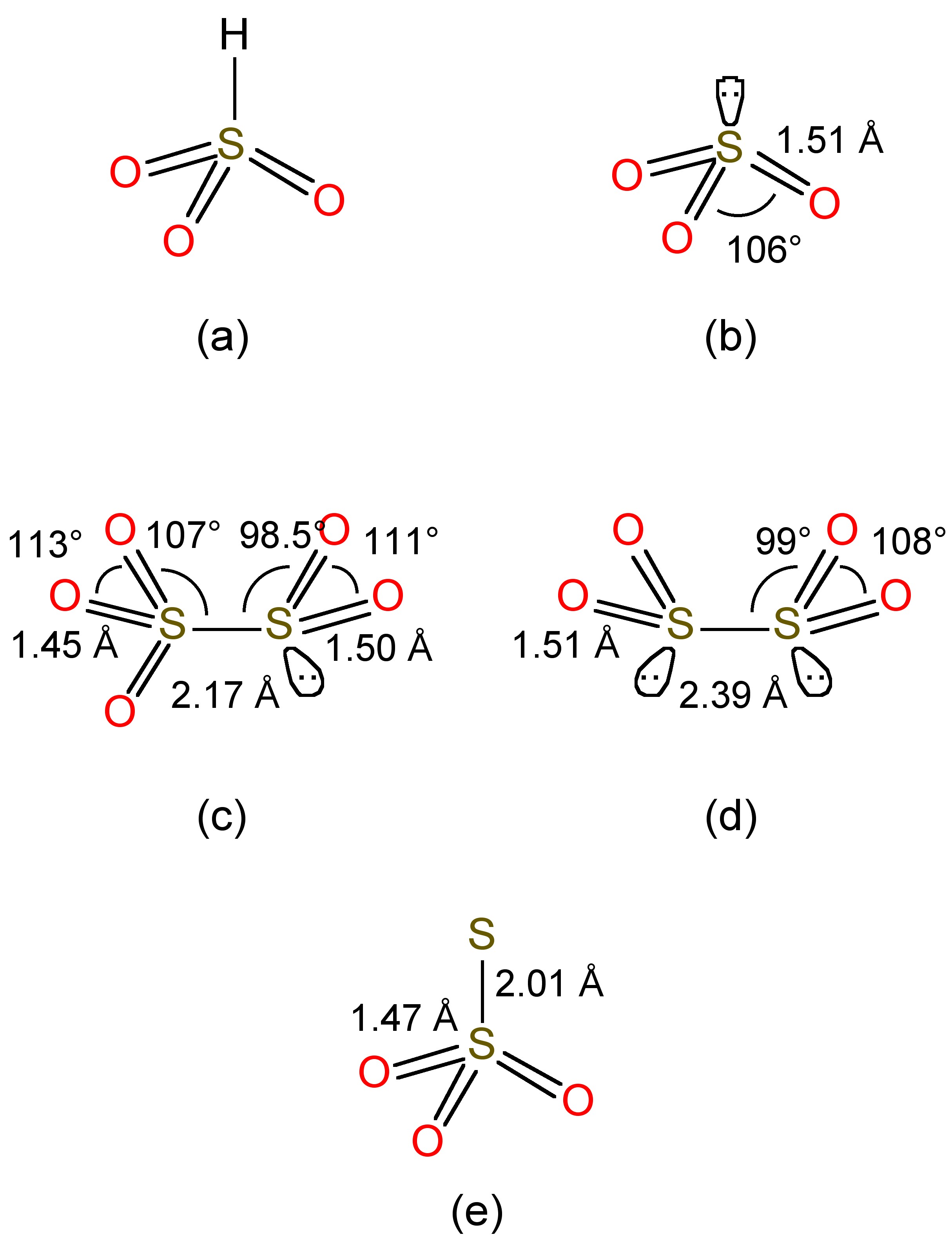
Bisulfite undergoes a further equilibrium, (9.8.3), to form disulfite, whose structure is shown in Figure \(\PageIndex{2}\)c.
\[ \rm 2 HSO_3^- \rightleftharpoons S_2O_5^{2-} + H_2O \]
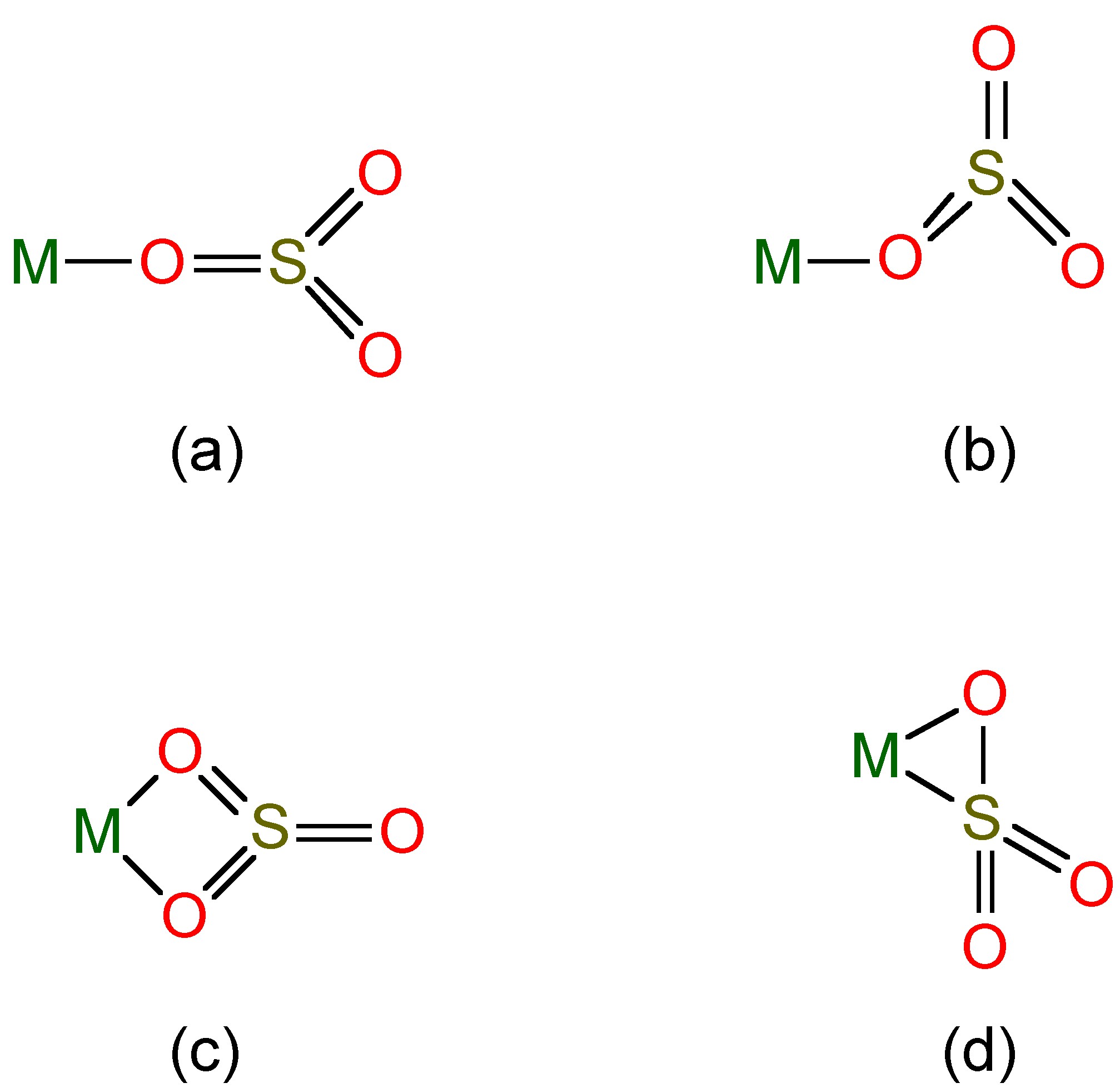
Salts of these anions are known, and complexes of the sulfite ion are known (Figure \(\PageIndex{3}\)), while SO2 itself can act as a ligand to heavy metals.
The bisulfite ion has strong reducing properties, e.g., (9.8.4) and (9.8.5).
\[ \rm 2 Fe^{3+} + SO_3^{2-} + 2 OH^- \rightarrow 2 Fe^{2+} + H_2O + SO_4^{2-}\]
\[ \rm 2 MnO_4^+ + 5 SO_3^{2-} + 6 H^+ \rightarrow 2 Mn^{2+} + 3 H_2O + 5 SO_4^{2-}\]
Bisulfite is also reduced by zinc in the presence of additional SO2, (9.8.6), to form the highly reducing dithionite anion (Figure \(\PageIndex{2}\)d). Reaction of bisulfite with elemental sulfur yields the thiosulfate anion (Figure \(\PageIndex{2}\)e), (9.8.7)f.
\[ \rm SO_3^{2-} + SO_2 \xrightarrow{Zn} S_2O_4^{2-}\]
\[ \rm SO_3^{2-} + S \rightarrow S_2O_3^{2-}\]
Sulfur trioxide and sulfuric acid
Oxidation of sulfur dioxide in the presence of a catalyst (e.g., platinum) yields sulfur trioxide, (9.8.8), which may be condensed to a liquid at room temperature (Bp = 45 °C).
\[ \rm 2 SO_2 + O_2 \xrightarrow{Pt} 2 SO_3\]
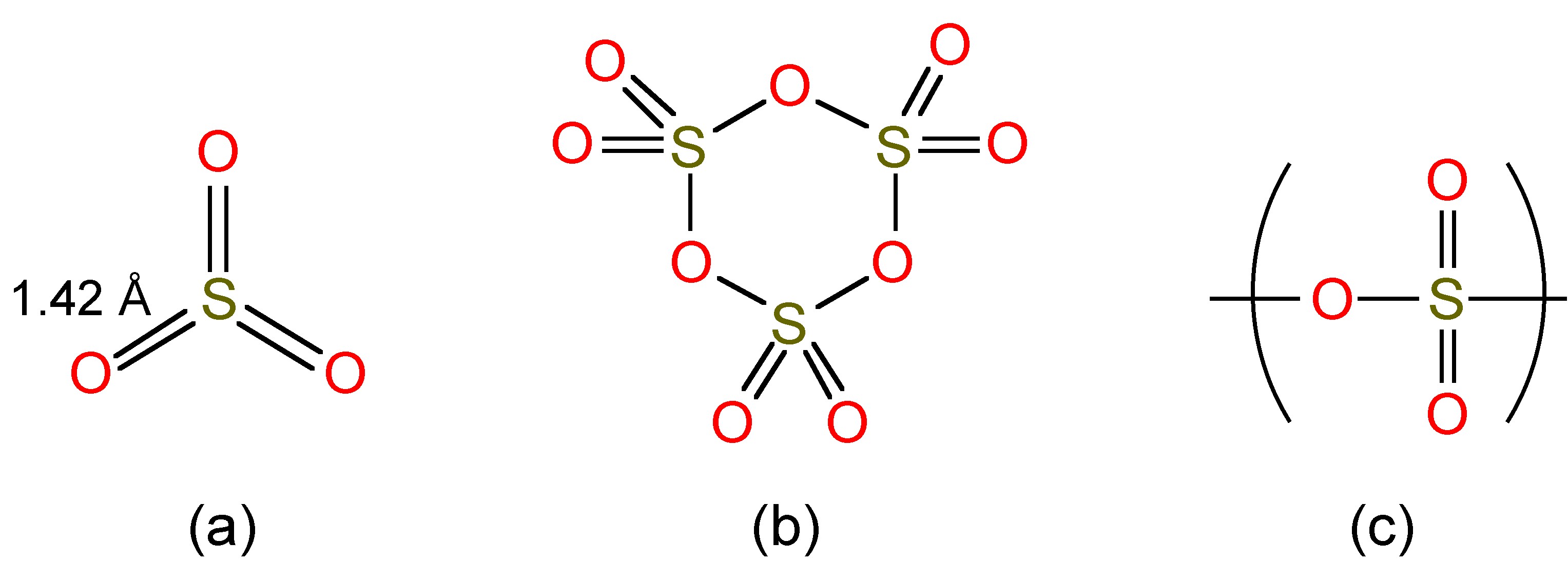
Liquid SO3 exists as a mixture of monomer and trimers (Figure \(\PageIndex{4}\)a and 4b), while as a solid (Mp = 16.9 °C) it forms polymers (Figure \(\PageIndex{4}\)c).
The reaction of SO3 with water results in the formation of sulfuric acid, H2SO4, as a viscous, hydrogen bonded liquid. Sulfuric acid is a strong protic acid, which in dilute solutions (in water) reacts as a dibasic acid, (9.8.9), forming bisulfate (HSO4-) and sulfate (SO42-) anions. A large number of salts are known for both anions. In addition, sulfate is known to act as a monodentate or bidentate ligand in coordination complexes.
\[ \rm H_2SO_4 \xrightleftharpoons[+ H^+]{- H^+} HSO_4^- \xrightleftharpoons[+ H^+]{- H^+} SO_4^{2-}\]
The dissolution of SO3 in concentrated sulfuric acid yields very corrosive, fuming sulfuric acid, which contains some pyrosulfuric acid, (9.8.10).
\[ \rm H_2SO_4 + SO_3 \rightleftharpoons H_2S_2O_7\]
WARNING
The corrosive properties of sulfuric acid are accentuated by its highly exothermic reaction with water. Burns from sulfuric acid are potentially more serious than those of comparable strong acids (e.g., hydrochloric acid), as there is additional tissue damage due to dehydration and particularly secondary thermal damage due to the heat liberated by the reaction with water.
Sulfur as a source of atmospheric pollution and acid rain
Sulfur dioxide is formed as a pollutant during the combustion of sulfur containing fuels, in particular coal. While the emission of SO2 itself leads to concerns it is its conversion to sulfuric acid in the form of acid rain that has been of concern for several decades. The pathway for the formation of sulfuric acid in the atmosphere is depandant on whether the reaction occurs in dry atmosphere or in clouds and rain.
Gaseous reactions in a dry atmosphere
In the dry atmosphere, gaseous sulfur dioxide reacts with the hydroxide radical (formed by the photochemical decomposition of ozone, (9.8.11) and (9.8.12), in the presence of a non-reactive gas molecule such as nitrogen, (9.8.12). The sulfurous acid, thus formed reacts with oxygen to generate sulfur trioxide, (9.8.13), which reacts with water to form sulfuric acid, (9.8.14).
\[ \rm O_3 + h\nu \rightarrow O* + O_2\]
\[ \rm O* + H_2O \rightarrow 2 HO\cdot\]
\[ \rm HSO_3 + O_2 \rightarrow HO_2 + SO_3\]
\[ \rm SO_3 + H_2O \rightarrow H_2SO_4\]
Measurements indicate that the conversion rate of SO2 to H2SO4 is 4% per hour on a clear sunny day, but the rate is slower during the winter.
Liquid phase reactions in clouds and rain
In the liquid phase SO2 reacts directly with water, (9.8.15). The bisulfite (HSO3-) is oxidized by hydrogen peroxide forming a forming bisulfate (HSO4-) solution, (9.8.16).
\[ \rm 2 SO_2 + 2 H_2O \rightarrow SO_3^{2-} + HSO_3^- + 3 H^+\]
\[ \rm HSO_3^- + H_2O_2 \rightarrow HSO_4^- + H_2O\]
Water soluble hydrogen peroxide is formed by the oxidation of water, (9.8.17).
\[ \rm HO_2 + HO_2 \rightarrow H_2O_2 + O_2\]
The HO2 radical is formed by the photolysis of organic carbonyl compounds, e.g., formaldehyde in (9.8.18) and (9.8.19).
\[ \rm H_2\text{C=O} + h\nu \rightarrow H\cdot + HCO\cdot\]
\[ \rm HCO + O_2 \rightarrow HO_2 + CO\]
The conversion rate is independent of pH is very fast: almost 100% per hour in summer. However, the conversion is limited by the supply of hydrogen peroxide, which is often present in much lower levels than SO2. Thus, a reduction in sulfur dioxide emissions does not always correlate with a reduction of wet acid deposition.


