1.10: The Origins of Atoms and Elements
- Page ID
- 56510
The Earth is composed of 90 chemical elements, of which 81 have at least one stable isotope. Most of these elements have also been detected in stars. Where did these elements come from? The accepted scenario is that the first major element to condense out of the primordial soup was helium, which still comprises about one-quarter of the mass of the known universe.
Stellar nucleosynthesis is the generation of new elements by nuclear reaction within stars. According to the Big bang theory for which there is now overwhelming evidence, the universe as we know it had its origin in a point source or singularity that began an explosive expansion about 12-15 billion years ago, and which is still continuing. Following a brief period of extremely rapid expansion. Helium and hydrogen became stable during the first few minutes, along with some of the very lightest nuclides up to lithium, which were formed through various nuclear reaction. Formation of most heavier elements was delayed for about million years until nucleosynthesis commenced in the first stars. Hydrogen still accounts for about 93% of the atoms in the universe.
All elements beyond hydrogen were formed in regions where the concentration of matter was large, and the temperature was high; in other words, in stars. The formation of a star begins when the gravitational forces due to a large local concentration of hydrogen bring about a contraction and compression to densities of around 105 g cm–3. This is a highly exothermic process in which the gravitational potential energy is released as heat, about 1200 kJ per gram, raising the temperature to about 107 K. Under these conditions, hydrogen nuclei possess sufficient kinetic energy to overcome their electrostatic repulsion and undergo nuclear fusion to generate helium (Figure \(\PageIndex{1}\)). This is known as “hydrogen burning”.
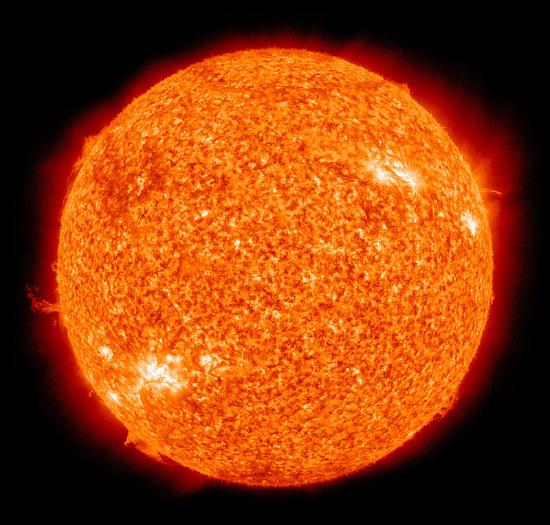
As hydrogen burning proceeds, the helium collects in the core of the star, raising the density to 108 g cm–3 and the temperature to 108 K. This temperature is high enough to initiate helium burning. The size of a star depends on the balance between the kinetic energy of its matter and the gravitational attraction of its mass. As the helium burning runs its course, the temperature drops and the star begins to contract. The course of further nucleosynthesis event and the subsequent fate of the star itself depends on the star’s mass. Fusion into heavier species than iron is also precluded by the electrostatic repulsion of the highly charged nuclei. However,further nuclear processes are responsible for these.
Contributors and Attributions
Stephen Lower, Professor Emeritus (Simon Fraser U.) Chem1 Virtual Textbook

