1.3: The Scientific Approach to Knowledge
- Page ID
- 25455
- Give a short history of the concept of the atom.
- Describe the contributions of Democritus and Dalton to atomic theory.
- Summarize Dalton's atomic theory and explain its historical development.
We discussed the particulate model of matter, but this is not a clear conclusion to the casual observer of nature. While all modern scientists accept the concept of the atom, when the concept of the atom was first proposed about 2,500 years ago, ancient philosophers laughed at the idea. It has always been difficult to convince people of the existence of things that are too small to see. We will spend some time considering the evidence (observations) that convince scientists of the existence of atoms.
About 2,500 years ago, early Greek philosophers believed the entire universe was a single, huge, entity. In other words, "everything was one." They believed that all objects, all matter, and all substances were connected as a single, big, unchangeable "thing." One of the first people to propose "atoms" was a man known as Democritus. As an alternative to the beliefs of the Greek philosophers, he suggested that atomos, or atomon - tiny, indivisible, solid objects - make up all matter in the universe.


Democritus then reasoned that changes occur when the many atomos in an object were reconnected or recombined in different ways. Democritus even extended this theory, suggesting that there were different varieties of atomos with different shapes, sizes, and masses. He thought, however, that shape, size, and mass were the only properties differentiating the different types of atomos. According to Democritus, other characteristics, like color and taste, did not reflect properties of the atomos themselves, but rather, resulted from the different ways in which the atomos were combined and connected to one another.
The early Greek philosophers tried to understand the nature of the world through reason and logic, but not through experiment and observation. As a result, they had some very interesting ideas, but they felt no need to justify their ideas based on life experiences. In a lot of ways, you can think of the Greek philosophers as being "all thought and no action." It's truly amazing how much they achieved using their minds, but because they never performed any experiments, they missed or rejected a lot of discoveries that they could have made otherwise. Greek philosophers dismissed Democritus' theory entirely. Sadly, it took over two millennia before the theory of atomos (or "atoms," as they're known today) was fully appreciated.
Greek philosophers were "all thought and no action" and did not feel the need to test their theories with reality. In contrast, Dalton's efforts were based on experimentation and testing ideas against reality.
While it must be assumed that many more scientists, philosophers, and others studied composition of matter after Democritus, a major leap forward in our understanding of the composition of matter took place in the 1800's with the work of the British scientists John Dalton. His atomic theory is a fundamental concept that states that all elements are composed of atoms. Dalton formulated his theory by focusing on experimental results (in contrast to the ancient Greek philosophers) by studied the weights of various elements and compounds. From his experiments and observations, as well as the work from peers of his time, Dalton proposed a new theory of the atom.The general tenets of this theory were as follows:
- All matter is composed of extremely small particles called atoms.
- Atoms of a given element are identical in size, mass, and other properties. Atoms of different elements differ in size, mass, and other properties.
- Atoms cannot be subdivided, created, or destroyed.
- Atoms of different elements can combine in simple whole number ratios to form chemical compounds.
- In chemical reactions, atoms are combined, separated, or rearranged.
Dalton's atomic theory has been largely accepted by the scientific community, with the exception of three changes. We know now that (1) an atom can be further subdivided, (2) all atoms of an element are not identical in mass, and (3) using nuclear fission and fusion techniques, we can create or destroy atoms by changing them into other atoms.
The evidence for atoms is so great that few doubt their existence. In fact, individual atoms are now routinely observed with state-of-the art technologies.
The Scientific Method
Scientists search for answers to questions and solutions to problems by using a procedure called the scientific method. This procedure consists of making observations, formulating hypotheses, and designing experiments, which in turn lead to additional observations, hypotheses, and experiments in repeated cycles (Figure \(\PageIndex{2}\)).
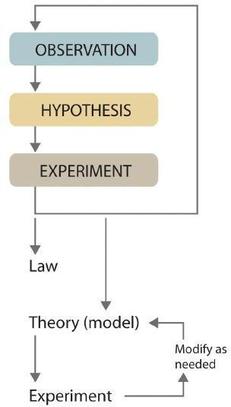
Observations can be qualitative or quantitative. Qualitative observations describe properties or occurrences in ways that do not rely on numbers. Examples of qualitative observations include the following: the outside air temperature is cooler during the winter season, table salt is a crystalline solid, sulfur crystals are yellow, and dissolving a penny in dilute nitric acid forms a blue solution acid forms a blue solution and a brown gas. After deciding to learn more about an observation or a set of observations, scientists generally begin an investigation by forming a hypothesis, a tentative explanation for the observation(s). The hypothesis may not be correct, but it puts the scientist’s understanding of the system being studied into a form that can be tested. After a hypothesis has been formed, scientists conduct experiments to test its validity. Experiments are systematic observations or measurements, preferably made under controlled conditions—that is, under conditions in which a single variable changes. For example, in the dinosaur extinction scenario, iridium concentrations in the dinosaur extinction scenario, iridium concentrations were measured worldwide and compared. A properly designed and executed experiment enables a scientist to determine whether the original hypothesis is valid. Experiments often demonstrate that the hypothesis is incorrect or that it must be modified. More experimental data are then collected and analyzed, at which point a scientist may begin to think that the results are sufficiently reproducible (i.e., dependable) to merit being summarized in a law, a verbal or mathematical description of a phenomenon that allows for general predictions. A law simply says what happens; it does not address the question of why.
It is important to remember that scientists have a tendency to formulate hypotheses in familiar terms simply because it is difficult to propose something that has never been encountered or imagined before. As a result, scientists sometimes discount or overlook unexpected findings that disagree with the basic assumptions behind the hypothesis or theory being tested. Fortunately, truly important findings are immediately subject to independent verification by scientists in other laboratories, so science is a self-correcting discipline.
Fundamental Definitions in Chemistry: https://youtu.be/SBwjbkFNkdw
Measurementsunderlie the Scientific Method
The results of a scientific experiment must be communicated to be of value. This affords an opportunity for other scientists to check them. It also allows the scientific community, and sometimes the general public, to share new knowledge. Communication, however, is not always as straightforward as it might seem. Ambiguous terminology can often turn a seemingly clear statement into a morass of misunderstanding.
As an example, consider someone tells you that the forecast is a high of 25°. Do you put on a winter jacket, or summer wear? If you are thinking winter, then you interpreted the temperature as 25°F. However if 25°C was meant, which is equal to 77°F, a winter jacket would be far too warm. Or consider filling up a car with gasoline. If you are in the US, you will be dealing in dollars per gallon, whereas, if you were in continental Europe, you will be dealing in Euros per liter. Given that the exchange rate from USD to Euros fluctuates and that there are roughly 3.79 liters in 1 gallon, it is difficult to simply compare numbers between gas prices in the USA, and say, France, if you don't know what units you are using. As a final example, consider the speed 24 meters/sec. Do you interpret this as close to highway speed limits in the US? It is the same speed as 53.7 miles per hour, likely a more familiar set of units for speed for people in the US.
Scientists are not all that different from other people—they too have favorite units which are especially suited to certain areas of research. Nevertheless, scientists have constantly pressed for improvement and uniformity in systems of measurement. The first such action occurred nearly 200 years ago when, in the aftermath of the French Revolution, the metric system spread over most of continental Europe and was adopted by scientists everywhere. The United States nearly followed suit, but in 1799 Thomas Jefferson was unsuccessful in persuading Congress that a system based on powers of 10 was far more convenient and would eventually become the standard of the world.
The metric system has undergone continuous evolution and improvement since its original adoption by France. Beginning in 1899, a series of international conferences have been held for the purpose of redefining and regularizing the system of units. In 1960 the Eleventh Conference on Weights and Measures proposed major changes in the metric system and suggested a new name — the International System of Units — for the revised metric system. (The abbreviation SI, from the French Système International, is commonly used.) Scientific bodies such as the U.S. National Bureau of Standards and the International Union of Pure and Applied Chemistry have endorsed the SI.
Summary
- Chemists expand their knowledge by making observations, carrying out experiments, and testing hypotheses to develop laws to summarize their results and theories to explain them. In doing so, they are using the scientific method.
- 2,500 years ago, Democritus suggested that all matter in the universe was made up of tiny, indivisible, solid objects he called "atomos." However, other Greek philosophers disliked Democritus' "atomos" theory because they felt it was illogical. Dalton's Atomic Theory is the first scientific theory to relate chemical changes to the structure, properties, and behavior of the atom.
- The natural sciences begin with observation, and this usually involves numerical measurements of quantities such as length, volume, density, and temperature. Most of these quantities have units of some kind associated with them, and these units must be retained when you use them in calculations. Measuring units can be defined in terms of a very small number of fundamental ones that, through "dimensional analysis", provide insight into their derivation and meaning, and must be understood when converting between different unit systems.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:

