4.E: Reactions in Aqueous Solutions (Exercises)
- Page ID
- 19108
These are homework exercises to accompany the Textmap created for "Chemistry: Principles, Patterns, and Applications" by Bruce A. Averill and Patricia Eldredge. Complementary General Chemistry question banks can be found for other Textmaps and can be accessed here. In addition to these publicly available questions, access to private problems bank for use in exams and homework is available to faculty only on an individual basis; please contact Delmar Larsen for an account with access permission.
Application Problems
Please be sure you are familiar with the topics discussed in Essential Skills 3 (Section 4.1 "Aqueous Solutions"0) before proceeding to the Application Problems.Problems marked with a ♦ involve multiple concepts.
-
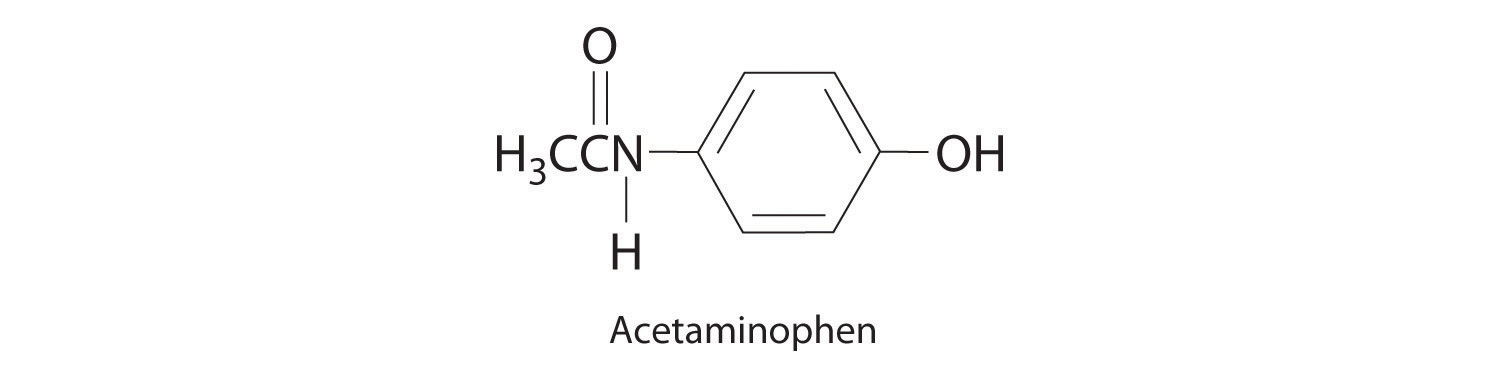
♦ Acetaminophen (molar mass = 151 g/mol) is an analgesic used as a substitute for aspirin. If a child’s dose contains 80.0 mg of acetaminophen/5.00 mL of an ethanol-water solution, what is the molar concentration? Acetaminophen is frequently packaged as an ethanol-water solution rather than as an aqueous one. Why?
-
♦ Lead may have been the first metal ever recovered from its ore by humans. Its cation (Pb2+) forms a precipitate with Cl− according to the following equation:
Pb2+(aq) + 2Cl−(aq) → PbCl2(s)When PbCl2 is dissolved in hot water, its presence can be confirmed by its reaction with CrO42−, with which it forms a yellow precipitate:
Pb2+(aq) + CrO42−(aq) → PbCrO4(s)The precipitate is used as a rust inhibitor and in pigments.
- What type of reaction does each equation represent?
- If 100 mL of a Pb2+ solution produce 1.65 g of lead chromate, what was the concentration of the lead solution?
- What volume of a potassium chromate solution containing 0.503 g of solute per 250.0 mL is needed for this reaction?
- If all the PbCrO4 originated from PbCl2, what volume of a 1.463 M NaCl solution was needed for the initial reaction?
- Why is there environmental concern over the use of PbCrO4?
-
♦ Reactions that affect buried marble artifacts present a problem for archaeological chemists. Groundwater dissolves atmospheric carbon dioxide to produce an aqueous solution of carbonic acid:
CO2(g) + H2O(l) → H2CO3(aq)This weakly acidic carbonic acid solution dissolves marble, converting it to soluble calcium bicarbonate:
CaCO3(s) + H2CO3(aq) → Ca(HCO3)2(aq)Evaporation of water causes carbon dioxide to be driven off, resulting in the precipitation of calcium carbonate:
Ca(HCO3)2(aq) → CaCO3(s) + H2O(l) + CO2(g)The reprecipitated calcium carbonate forms a hard scale, or incrustation, on the surface of the object.
- If 8.5 g of calcium carbonate were obtained by evaporating 250 mL of a solution of calcium bicarbonate followed by drying, what was the molarity of the initial calcium bicarbonate solution, assuming complete reaction?
- If the overall reaction sequence was 75% efficient, how many grams of carbonic acid were initially dissolved in the 250 mL to produce the calcium bicarbonate?
-
How many Maalox tablets are needed to neutralize 5.00 mL of 0.100 M HCl stomach acid if each tablet contains 200 mg Mg(OH)2 + 200 mg Al(OH)3? Each Rolaids tablet contains 412 mg CaCO3 + 80.0 mg Mg(OH)2. How many Rolaids tablets are needed? Suggest another formula (and approximate composition) for an effective antacid tablet.
-
Citric acid (C6H8O7, molar mass = 192.12 g/mol) is a triprotic acid extracted from citrus fruits and pineapple waste that provides tartness in beverages and jellies. How many grams of citric acid are contained in a 25.00 mL sample that requires 38.43 mL of 1.075 M NaOH for neutralization to occur? What is the formula of the calcium salt of this compound?
-
♦ A method for determining the molarity of a strongly acidic solution has been developed based on the fact that a standard solution of potassium iodide and potassium iodate yields iodine when treated with acid:
IO3− + 5I− + 6H+ → 3I2 + 3H2OStarch is used as the indicator in this titration because starch reacts with iodine in the presence of iodide to form an intense blue complex. The amount of iodine produced from this reaction can be determined by subsequent titration with thiosulfate:
2S2O32+ + I2 → S4O62− + 2I−The endpoint is reached when the solution becomes colorless.
- The thiosulfate solution was determined to be 1.023 M. If 37.63 mL of thiosulfate solution were needed to titrate a 25.00 mL sample of an acid, what was the H+ ion concentration of the acid?
- If the 25.00 mL sample that was titrated had been produced by dilution of a 10.00 mL sample of acid, what was the molarity of the acid in the original solution?
- Why might this be an effective method for determining the molarity of a strong acid, such as H2SO4?
-
♦ Sewage processing occurs in three stages. Primary treatment removes suspended solids, secondary treatment involves biological processes that decompose organic matter, and tertiary treatment removes specific pollutants that arise from secondary treatment (generally phosphates). Phosphate can be removed by treating the HPO42− solution produced in the second stage with lime (CaO) to precipitate hydroxyapatite [Ca5(PO4)3OH].
- Write a balanced chemical equation for the reaction that occurs in the tertiary treatment process.
- What has been neutralized in this process?
- Four pounds of hydroxyapatite precipitated from the water. What mass of lime was used in the reaction?
- Assuming a volume of water of 30 m3, what was the hydrogen phosphate anion concentration in the water?
-
Calcium hydroxide and calcium carbonate are effective in neutralizing the effects of acid rain on lakes. Suggest other compounds that might be effective in treating lakes. Give a plausible reason to explain why Ca(OH)2 and CaCO3 are used.
-
♦ Approximately 95% of the chlorine produced industrially comes from the electrolysis of sodium chloride solutions (brine):
NaCl(aq) + H2O(l) → Cl2(g) + H2(g) + NaOH(aq)Chlorine is a respiratory irritant whose presence is easily detected by its penetrating odor and greenish-yellow color. One use for the chlorine produced is in the manufacture of hydrochloric acid.
- In the chemical equation shown, what has been oxidized and what has been reduced?
- Write the oxidation and reduction equations for this reaction.
- Balance the net ionic equation.
- Name another salt that might produce chlorine by electrolysis and give the expected products. Identify those products as gases, liquids, or solids.
-
♦ The lead/acid battery used in automobiles consists of six cells that produce a 12 V electrical system. During discharge, lead(IV) oxide, lead, and aqueous sulfuric acid react to form lead(II) sulfate and water.
- What has been oxidized and what has been reduced?
- Write and balance the equation for the reaction.
- What is the net ionic equation?
- What is the complete ionic equation?
- What hazard is associated with handling automobile batteries?
-
♦ The use of iron, which is abundant in Earth’s crust, goes back to prehistoric times. In fact, it is believed that the Egyptians used iron implements approximately 5000 years ago. One method for quantifying the iron concentration in a sample involves three steps. The first step is to dissolve a portion of the sample in concentrated hydrochloric acid to produce ferric chloride; the second is to reduce Fe3+ to Fe2+ using zinc metal; and the third is to titrate Fe2+ with permanganate, producing Mn2+(aq) and ferric iron in the form of Fe2O3.
- Write chemical equations for all three steps.
- Write the net ionic equations for these three reactions.
- If 27.64 mL of a 1.032 M solution of permanganate are required to titrate 25.00 mL of Fe2+ in the third step, how many grams of Fe were in the original sample?
- Based on your answer to part c, if the original sample weighed 50.32 g, what was the percentage of iron?
-
Baking powder, which is a mixture of tartaric acid and sodium bicarbonate, is used in baking cakes and bread. Why does bread rise when you use baking powder? What type of reaction is involved?
-
An activity series exists for the halogens, which is based on the ease of reducing the diatomic halogen molecule (X2) to X−. Experimentally, it is found that fluorine is the easiest halogen to reduce (i.e., F2 is the best oxidant), and iodine is the hardest halogen to reduce (i.e., I2 is the worst oxidant). Consequently, the addition of any diatomic halogen, Y2, to solutions containing a halide ion (X−) that lies below Y in the periodic table will result in the reduction of Y2 to Y− and the oxidation of X−. Describe what you would expect to occur when
- chlorine is added to an aqueous solution of bromide.
- iodine crystals are added to a solution of potassium bromide.
Bromide is present in naturally occurring salt solutions called brines. Based on your answers, propose an effective method to remove bromide from brine.
-
♦ Marble is composed of mostly calcium carbonate. Assuming that acid rain contains 4.0 × 10−5 M H2SO4, approximately what volume of rain is necessary to dissolve a 250 lb marble statue?
-
♦ One of the “first-aid” measures used to neutralize lakes whose pH has dropped to critical levels is to spray them with slaked lime (Ca(OH)2) or limestone (CaCO3). (A slower but effective alternative is to add limestone boulders.) How much slaked lime would be needed to neutralize the acid in a lake that contains 4.0 × 10−5 M H2SO4 and has a volume of 1.2 cubic miles (5.0 × 1012 L)?
-
Recall from Section 4.3 "Stoichiometry of Reactions in Solution" that the reaction of ethanol with dichromate ion in acidic solution yields acetic acid and Cr3+(aq):
Cr2O72−(aq) + CH3CH2OH(aq) → Cr3+(aq) + CH3CO2H(aq)Balance the equation for this reaction using oxidation states. (Hint: the oxidation state of carbon in the CH3 group remains unchanged, as do the oxidation states of hydrogen and oxygen.)
Answers
-
0.106 M acetaminophen; acetaminophen is an organic compound that is much more soluble in ethanol than water, so using an ethanol/water mixture as the solvent allows a higher concentration of the drug to be used.
-
-
- 0.34 M Ca(HCO3)2
- 7.0 g H2CO3
-
-
2.646 g citric acid, Ca3(C6H5O7)2
-
-
- 5CaO + 3HPO42− + 2H2O → Ca5(PO4)3OH + 6OH−
- This is an acid–base reaction, in which the acid is the HPO42− ion and the base is CaO. Transferring a proton from the acid to the base produces the PO43− ion and the hydroxide ion.
- 2.2 lbs (1 kg) of lime
- 3.6 × 10−4 M HPO42−
-
-
- Chloride is oxidized, and protons are reduced.
-
Oxidation: 2Cl− → Cl2 + 2e− Reduction: 2H2O + 2e− → H2 + 2OH− -
2Cl− + 2H2O → Cl2 + H2 + 2OH−


