8.2.2B: Solutions of Gaseous Solutes in Liquid Solvents
- Page ID
- 78395
Make sure you thoroughly understand the following essential ideas:
- Explain why, in contrast, gases tend to be only slightly soluble in liquids and solids. And why are some combinations, such as the dissolution of ammonia or hydrogen chloride in water, significant exceptions to this rule?
- State Henry's law and explain
- Why do fish in rivers and streams sometimes become asphyxiated (oxygen-starved) in hot weather?
Solutions of Gases in Liquids
Gases dissolve in liquids, but usually only to a small extent. When a gas dissolves in a liquid, the ability of the gas molecules to move freely throughout the volume of the solvent is greatly restricted. If this latter volume is small, as is often the case, the gas is effectively being compressed. Both of these effects amount to a decrease in the entropy of the gas that is not usually compensated by the entropy increase due to mixing of the two kinds of molecules. Such processes greatly restrict the solubility of gases in liquids.
| liquid solvent, solute → | gas |
|---|---|
| energy to disperse solute | nil |
| energy to introduce into solvent | medium to large |
| increase in entropy | negative |
| miscibility | usually very limited |
Solubility of gases in water
One important consequence of the entropy decrease when a gas dissolves in a liquid is that the solubility of a gas decreases at higher temperatures; this is in contrast to most other situations, where a rise in temperature usually leads to increased solubility. Bringing a liquid to its boiling point will completely remove a gaseous solute. Some typical gas solubilities, expressed in the number of moles of gas at 1 atm pressure that will dissolve in a liter of water at 25° C, are given below:
| solute | formula | solubility, mol L–1 atm–1 |
|---|---|---|
| ammonia | NH3 | 57 |
| carbon dioxide | CO2 | 0.0308 |
| methane | CH4 | 0.00129 |
| nitrogen | N2 | 0.000661 |
| oxygen | O2 | 0.00126 |
| sulfur dioxide | SO2 | 1.25 |
As we indicated above, the only gases that are readily soluble in water are those whose polar character allows them to interact strongly with it.
Ammonia is remarkably soluble in water
Inspection of the above table reveals that ammonia is a champion in this regard. At 0° C, one liter of water will dissolve about 90 g (5.3 mol) of ammonia. The reaction of ammonia with water according to
\[\ce{NH_3 + H_2O → NH_4^{+} + OH^{–}}\]
makes no significant contribution to its solubility; the equilibrium lies heavily on the left side (as evidenced by the strong odor of ammonia solutions). Only about four out of every 1000 NH3molecules are in the form of ammonium ions at equilibrium. This is truly impressive when one calculates that this quantity of NH3 would occupy (5.3 mol) × (22.4 L mol–1) = 119 L at STP. Thus one volume of water will dissolve over 100 volumes of this gas. It is even more impressive when you realize that in order to compress 119 L of an ideal gas into a volume of 1 L, a pressure of 119 atm would need to be applied! This, together with the observation that dissolution of ammonia is accompanied by the liberation of a considerable amount of heat, tells us that the high solubility of ammonia is due to the formation of more hydrogen bonds (to H2O) than are broken within the water structure in order to accommodate the NH3 molecule.
If we actually compress 90 g of pure NH3 gas to 1 L, it will liquefy, and the vapor pressure of the liquid would be about 9 atm. In other words, the escaping tendency of NH3 molecules from H2O is only about 1/9th of what it is from liquid NH3. One way of interpreting this is that the strong intermolecular (dipole-dipole) attractions between NH3 and the solvent H2O give rise to a force that has the effect of a negative pressure of 9 atm.
Solubility of gases decreases with Temperature
Recall that entropy is a measure of the ability of thermal energy to spread and be shared and exchanged by molecules in the system. Higher temperature exerts a kind of multiplying effect on a positive entropy change by increasing the amount of thermal energy available for sharing. Have you ever noticed the tiny bubbles that form near the bottom of a container of water when it is placed on a hot stove? These bubbles contain air that was previously dissolved in the water, but reaches its solubility limit as the water is warmed. You can completely rid a liquid of any dissolved gases (including unwanted ones such as Cl2 or H2S) by boiling it in an open container.
This is quite different from the behavior of most (but not all) solutions of solid or liquid solutes in liquid solvents. The reason for this behavior is the very large entropy increase that gases undergo when they are released from the confines of a condensed phase .
Solubility of Oxygen in water
Fresh water at sea level dissolves 14.6 mg of oxygen per liter at 0°C and 8.2 mg/L at 25°C. These saturation levels ensure that fish and other gilled aquatic animals are able to extract sufficient oxygen to meet their respiratory needs. But in actual aquatic environments, the presence of decaying organic matter or nitrogenous runoff can reduce these levels far below saturation. The health and survival of these organisms is severely curtailed when oxygen concentrations fall to around 5 mg/L.
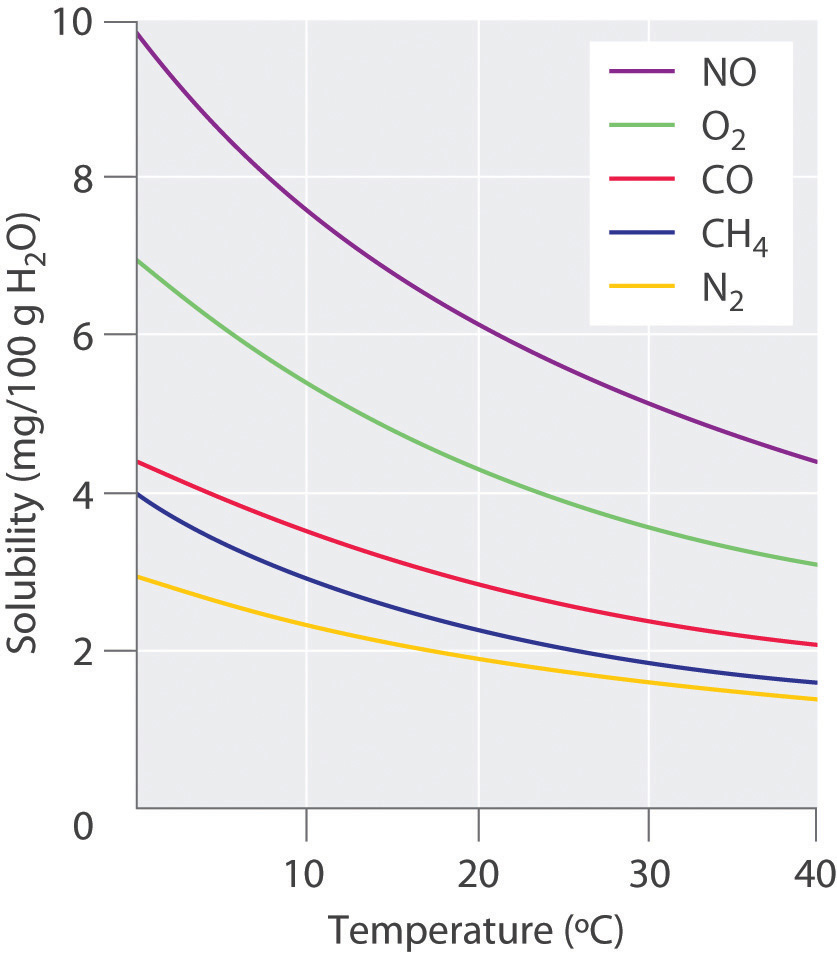
The temperature dependence of the solubility of oxygen in water is an important consideration for the well-being of aquatic life; thermal pollution of natural waters (due to the influx of cooling water from power plants) has been known to reduce the dissolved oxygen concentration to levels low enough to kill fish. The advent of summer temperatures in a river can have the same effect if the oxygen concentration has already been partially depleted by reaction with organic pollutants.
Solubility of gases increases with pressure: Henry's Law
The pressure of a gas is a measure of its "escaping tendency" from a phase. So it stands to reason that raising the pressure of a gas in contact with a solvent will cause a larger fraction of it to "escape" into the solvent phase. The direct-proportionality of gas solubility to pressure was discovered by William Henry (1775-1836) and is known as Henry's Law. It is usually written as
\[P = k_H C \label{7b.2.1}\]
with
- \(P\) is the partial pressure of the gas above the liquid,
- \(C\) is the concentration of gas dissolved in the liquid, and
- \(k_H\) is the Henry's law constant, which can be expressed in various units, and in some instances is defined in different ways, so be very careful to note these units when using published values.
For Table 7b.2.X, kH is given as
\[ k_H = \dfrac{\text{partial pressure of gas in atm}}{\text{concentration in liquid} \; mol \;L^{–1}}\]
| gas | He | N2 | O2 | CO2 | CH4 | NH3 |
|---|---|---|---|---|---|---|
| KH | 2703 | 1639 | 769 | 29.4 | 0.00129 | 57 |
Some vendors of bottled waters sell pressurized "oxygenated water" that is (falsely) purported to enhance health and athletic performance by supplying more oxygen to the body.
- How many moles of O2 will be in equilibrium with one liter of water at 25° C when the partial pressure of O2 above the water is 2.0 atm?
- How many mL of air (21% O2 v/v) must you inhale in order to introduce an equivalent quantity of O2 into the lungs (where it might actually do some good?)
Solution:
- Solving Henry's law for the concentration, we get
\[C = \dfrac{P}{k_H} = \dfrac{2.0\; atm}{769\; L\; atm \;mol^{–1}} = 0.0026\; mol\; L^{–1}\]
- At 25° C, 0.0026 mol of O2 occupies (22.4 L) × (.0026 mol) × (298/273) = 0.063 L. The equivalent volume of air would be (0.063 L) (.21) = 0.303 L. Given that the average tidal volume of the human lung is around 400 mL, this means that taking one extra breath would take in more O2 than is present in 1 L of "oxygenated water".
Carbonated beverages: the history of "Fizz-ics"
Artificially carbonated water was first prepared by Joseph Priestley (who later discovered oxygen) in 1767 and was commercialized in 1783 by Joseph Schweppe, a Swiss-German jeweler. Naturally-carbonated spring waters have long been reputed to have curative values, and these became popular tourist destinations in the 19th century. The term "seltzer water" derives from one such spring in Niederselters, Germany. Of course, carbonation produced by fermentation has been known since ancient times. The tingling sensation that carbonated beverages produce in the mouth comes from the carbonic acid produced when bubbles of carbon dioxide come into contact with the mucous membranes of the mouth and tongue:
\[CO_2 + H_2O → H_2CO_3\]


