4.4: Bonding in Nitrogen, Oxygen, and Fluorine
- Page ID
- 355282
Even though the bonding of hydrogen and carbon atoms can generate a remarkable array of molecules, the hydrocarbons are really rather boring (chemically). They take part in a rather limited range of reactions and would not, on their own, be expected to produce anything like life. Of course there are many other elements, and their properties add chemical complexity to molecular behavior. From the perspective of living systems two of the most interesting elements are nitrogen and oxygen. Carbon has six electrons (two core and four valence) and can form four bonds with neighboring atoms. Nitrogen has seven electrons: two core and five valence: \(1 \mathrm{s}^{2}, 2 \mathrm{s}^{2}, 2 \mathrm{p}_{\mathrm{x}} {}^{1}, 2 \mathrm{p}_{\mathrm{y}} {}^{1}, 2 \mathrm{p}_{\mathrm{z}} {}^{1}\). So if you are following the rules, you might well assume that nitrogen would be able to form five bonds (after all, it has five valence electrons). But when we look carefully, we never see a nitrogen atom making five bonds, and in all stable compounds it makes only three bonds. We can explain this observation in several ways. One factor is that nitrogen atoms are too small to support five centers of electron density around themselves because the bonds begin to overlap, which is destabilizing, just like we saw with bulky groups around a carbon. Another factor is that there are only four orbitals available in nitrogen in the second quantum shell. If nitrogen were to form five bonds it would have to use orbitals from the next quantum shell (3), but these orbitals are so high in energy that the energy required would not be offset by the energy released upon on bond formation. Together these factors mean that nitrogen, and in fact all elements in the second row of the periodic table, are limited to bonding arrangements with no more than four centers of electron density. As we will see later on, elements in the next row, such as phosphorus (\(\mathrm{P}\)) and sulfur (\(\mathrm{S}\)), are larger and have more available orbitals for bonding. These elements can form up to six centers of electron density.
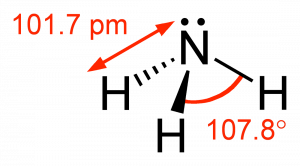
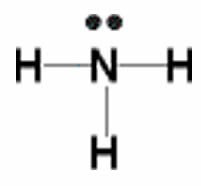
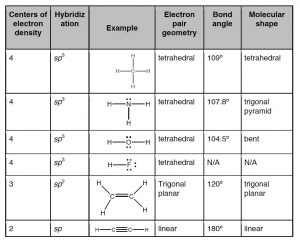
The simplest compound of nitrogen is molecular nitrogen, \(\mathrm{N}_{2}\). The two nitrogen atoms are bonded together by a triple bond, consisting of a \(\sigma\) and two \(\pi\) bonds. Molecular nitrogen, \(\mathrm{N}_{2}\)[8]A common nitrogen-containing molecule is ammonia (\(\mathrm{NH}_{3}\)), which is analogous to methane (\(\mathrm{CH}_{3}\)). In ammonia the nitrogen atom is bonded to three hydrogen atoms. These three bonds involve three of nitrogen’s valence electrons; the remaining two valence electrons occupy a non-bonding orbital and are referred to as a lone pair. Given the molecular hybridization orbital model that we are using this implies that four \(\mathrm{sp}^{3}\) orbitals are formed from the nitrogen atom’s \(2\mathrm{s}\) and \(2\mathrm{s}\) orbitals leading to four electron density centers around the nitrogen. The figure shows three representations of ammonia. The first indicates the \(\mathrm{N–H}\) bonds but fails to show the lone pair orbital. The second uses the wedge and dash convention and dots to illustrate the geometry of both bonds and the lone pair. The actual shape of the molecule is determined by the arrangements of electron clouds and the bonded atoms. In \(\mathrm{NH}_{3}\) all three bonds are equivalent (\(\mathrm{N–H}\)) and so must be symmetrical, but the lone pair orbital is different because it takes up more space than bonding pairs, can you imagine why? This has a subtle effect on the shape of the molecule. The angles between the \(\mathrm{C–H}\) bonds in \(\mathrm{CH}_{3}\) are equal and \(109^{\circ}\) while the angles between the \(\mathrm{N–H}\) bonds in \(\mathrm{NH}_{3}\) are slightly smaller, \(107.8^{\circ}\). The shape of the molecule itself (as outlined by the atoms) is a triangle-based pyramid rather than a tetrahedron. Finally the Lewis structure (the most abstract representation), indicates the bonds and lone pair electrons but gives an unrealistic depiction of the molecule’s geometry. It is up to the reader to supply the implicit information contained in the structure about bond angles and overall shape.
Bonding of Oxygen and Fluorine
Let us now consider oxygen (\(\mathrm{O}\)) which has eight electrons, two in the core and six valence (\(1 \mathrm{s}^{2}, 2 \mathrm{s}^{2}, 2 \mathrm{p}_{\mathrm{x}} {}^{1}, 2 \mathrm{p}_{\mathrm{y}} {}^{1}, 2 \mathrm{p}_{\mathrm{z}} {}^{1}\)). As with nitrogen, oxygen does not use all its electrons to form six bonds because it is too small and the orbitals that would need to be used to make six bonds are too high in energy to be energetically accessible; that is, not enough energy would be released upon bond formation to “pay for” that energy.
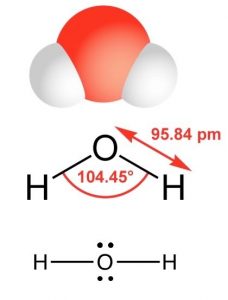
The simplest oxygen-containing molecule is molecular oxygen, \(\mathrm{O}_{2}\). On our simple covalent bond model the two oxygen atoms are connected by a \(\sigma\) and a \(\pi\) bond, forming a double bond.[9] The next simplest, stable, most common, and by far the most important compound of oxygen at least from the perspective of living organisms, is water (\(\mathrm{H}_{2}\mathrm{O}\)). In water there are two \(\mathrm{O-H}\) bonds and two lone pair non-bonding orbitals. As in the case of nitrogen, the orbitals are \(\mathrm{sp}^{3}\) hybrids and the oxygen atom is surrounded by four centers of electron density (see a pattern here?), two bonds, and two lone pairs. Again, the lone pair orbitals are larger than the \(\mathrm{O-H}\) bonding orbitals, which distorts the tetrahedral symmetry of the molecule. Instead of equal angles of \(109^{\circ}\) between the orbitals, the angle between the \(\mathrm{O-H}\) bonds is \(104.5^{\circ}\). When we use a Lewis structure to represent the structure of \(\mathrm{H}_{2}\mathrm{O}\), it is critical to include all valence shell electrons.
Continuing on across the periodic table we see that fluorine is the next element after oxygen. It has nine electrons: two core and seven valence. Rather than forming seven bonds fluorine only forms a single bond for basically the same reasons that oxygen only forms two bonds. Hydrogen fluoride, \(\mathrm{HF}\), has one bond, but four centers of electron density around the fluorine. Because \(\mathrm{HF}\) has only two atoms, they must by definition lie on a line and therefore we do not need to discuss its shape.
| Compound | Molar mass
(g/mole) |
Boiling point | Bond type | Bond length (pm) | Atomic radius
(pm) |
| \(\mathrm{CH}_{4}\) | \(16\) | \(–161^{\circ}\mathrm{C}\) | \(\mathrm{C–H}\) (in \(\mathrm{CH}_{4}\)) | \(109\) | \(\mathrm{C} – 70\) |
| \(\mathrm{NH}_{3}\) | \(17\) | \(–33^{\circ}\mathrm{C}\) | \(\mathrm{N–H}\) in (\(\mathrm{NH}_{3}\)) | \(101\) | \(\mathrm{N} – 65\) |
| \(\mathrm{H}_{2}\mathrm{O}\) | \(18\) | \(100^{\circ}\mathrm{C}\) | \(\mathrm{O–H}\) (in \(\mathrm{H}_{2}\mathrm{O}\)) | \(96\) | \(\mathrm{O} – 60\) |
| \(\mathrm{HF}\) | \(20\) | \(19.5^{\circ}\mathrm{C}\) | \(\mathrm{F–H}\) in (\(\mathrm{HF}\)) | \(92\) | \(\mathrm{F} – 50\) |
| \(\mathrm{Ne}\) | \(20\) | \(–246.08^{\circ}\mathrm{C}\) | not applicable | not applicable | \(\mathrm{Ne} – 38\) |
As we will see, a valid Lewis structure makes it possible to extrapolate a significant amount of information about a molecule’s chemical and physical properties. A confusing point is that the Lewis structure can be written in a number of apparently different ways, which are actually equivalent. The key to remember is that the Lewis structure does not attempt to depict a molecule’s actual three-dimensional structure. It is a shorthand (a “cartoon” if you like) that assumes you already know the arrangement of orbitals. No matter how it is drawn, the actual structure of a \(\mathrm{H}_{2}\mathrm{O}\) molecule is the same with a \(104.5^{\circ}\) bond angle between the \(\mathrm{O–H}\) bonds
| \(\mathrm{CH}_{4}\) | \(\mathrm{NH}_{3}\) | \(\mathrm{H}_{2}\mathrm{O}\) | \(\mathrm{HF}\) | \(\mathrm{Ne}\) |
| \(-258.7^{\circ} \mathrm{F}\left(-161.5^{\circ} \mathrm{C}\right)\) | \(-28.01^{\circ} \mathrm{F}\left(-33.34^{\circ} \mathrm{C}\right)\) | \(212^{\circ} \mathrm{F}\left(100^{\circ} \mathrm{C}\right)\) | \(67.1^{\circ} \mathrm{F}\left(19.5^{\circ} \mathrm{C}\right)\) | \(-410.9^{\circ} \mathrm{F}\left(-246.1^{\circ} \mathrm{C}\right)\) |
The tendency to form four centers (bonds or non-bonding pairs) has led to the rather misleading “octet rule”, which states that some elements tend to form molecules that have eight electrons around any atom (except for hydrogen). Unfortunately, the octet rule is far from being a rule because there are many exceptions, as we will see later. For example many of the elements past the second row of the periodic table are capable of bonding to more than four other atoms and some elements form stable compounds with less than eight electrons. It is important to remember that the octet rule is not the reason why atoms bond with each other, but it is a useful heuristic when constructing Lewis structures for the second row elements (\(\mathrm{C}\), \(\mathrm{N}\), \(\mathrm{O}\), \(\mathrm{F}\)).
Polarized Bonds and Electronegativity
Earlier we saw that the boiling points of hydrocarbons tend to increase as the number of carbons in the compound increases and that molecules with similar molecular weights have similar but not identical boiling points, with the shapes of the molecules having an effect, although a relatively small one. The attractions between hydrocarbons are due to London dispersion forces that depend on the size, surface area, and shape of the molecule. The larger these forces, the more strongly molecules will stick together and the more energy (higher temperature) will be needed to overcome these attractions.
Let us consider the boiling points of some common second row compounds involving bonds with hydrogen, that is, \(\mathrm{CH}_{4}\), \(\mathrm{NH}_{3}\), \(\mathrm{H}_{2}\mathrm{O}\) and \(\mathrm{HF}\), and neon (\(\mathrm{Ne}\)), which does not form bonds with hydrogen (the compounds of lithium, beryllium, and boron with hydrogen are much less common.) These compounds all have about the same molecular weight but different shapes. Based on our experiences with hydrocarbons, we would be well justified in predicting that they would have somewhat similar boiling points. Unfortunately, this prediction is not supported by experimental evidence (see Table). There is no clear trend, so something is going on that we have not yet considered. To explain this data we have to return to an idea that we discussed in Chapter \(3\), namely that the size of atoms decreases as you go across a row of the periodic table. Not only does the size (radius) of the atoms decrease (from \(70 \mathrm{~pm}\) for carbon to \(38 \mathrm{~pm}\) for neon) but so does the length of the bonds between the atoms and hydrogen (from \(109 \mathrm{~pm}\) to \(92 \mathrm{~pm}\)). This is both surprising and counterintuitive (which is why we are reminding you about it!)
Table \(4.4.1\) Electronegativities of Selected Elements
| \(\mathrm{H}\) | \(\mathrm{C}\) | \(\mathrm{N}\) | \(\mathrm{O}\) | \(\mathrm{F}\) |
| \(2.2\) | \(2.55\) | \(3.04\) | \(3.44\) | \(3.98\) |
Remember that the size of the atom is based on a balance between the attraction between the negatively charged electrons to the positively charged protons in the nucleus, the repulsions between the electrons as they get close to each other, and of course the arcane, but highly accurate rules of quantum mechanics. The reason that the atom’s size is decreasing as the number of protons increases is that each electron in the valence shell is attracted by an increasing number of protons in the nucleus. The more protons, the larger this attractive force. At the same time, the electrons in the same valence shell do not tend to repel each other as much as you might suspect because they are in different orbitals. Therefore the effective nuclear charge increases from left to right across the periodic table. This increase in effective nuclear charge doesn’t just affect the electrons in isolated atoms; it also affects the electrons in bonds. The ability to attract the electrons in bonds is called electronegativity, and because it derives from the same effect as that that determines effective nuclear charge and atomic radius, electronegativity also tends to increase from left to right across a row in the periodic table. It also decreases from top to bottom in a group of the periodic table. This makes sense because the further electrons are from the nucleus, the less they will be attracted to it. The exceptions to this rule are the noble gases (helium, neon, argon, etc.); because they do not form bonds with other elements (under normal circumstances) their electronegativities are usually not reported.
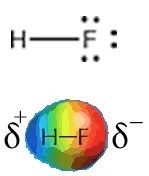
Fluorine is the most electronegative element and the Lewis structure of \(\mathrm{HF}\) shows one \(\mathrm{H–F}\) bond and three lone pairs. Fluorine attracts electrons very strongly—even the ones in the \(\mathrm{H–F}\) bond so that the fluorine atom ends up with more than its fair share of electrons and the hydrogen atom ends up with less. One way to think about this is that the electron density in the \(\mathrm{H–F}\) bond is shifted closer to the fluorine atom and away from the hydrogen atom (\(\rightarrow\)). The result of this is that the fluorine atom has more negative charge than positive charge and the hydrogen atom has more positive than negative charge. We indicate this by writing a \(\delta –\) charge on the fluorine atom and a \(\delta +\) charge on the hydrogen atom (\(\delta\) is often used to denote a small increment, that is less than 1). That means that there is an unequal distribution of charge in the molecule. The \(\mathrm{HF}\) molecule has a permanent dipole, that is, a separation of charge; the \(\mathrm{H–F}\) bond is said to be polarized and the molecule is considered polar. Permanent dipoles are different from the transient dipoles associated with London dispersion forces. Because of their permanent dipoles molecules of \(\mathrm{HF}\) interact with one another both attractively and repulsively, more strongly in some orientations than in others. \(\mathrm{HF}\) molecules are attracted to each other much more strongly than neon atoms, for example, because of the presence of these permanent dipoles. This results in a much higher boiling point for \(\mathrm{HF}\) than for neon (see above). That is, much more energy has to be supplied to the system to overcome the force of attraction and to separate \(\mathrm{HF}\) molecules from each other than is needed to separate neon atoms. An important point to note is that HF only has one bond, and the polarity of the bond is the same as the polarity of the whole molecule. As we will see, this is not the case in molecules with more complex structures.
It is relatively easy to predict whether a particular bond is polar by looking at the electronegativity differences between the atoms in that bond. Typically, elements on the left-hand side of the periodic table (metals) have rather low electronegativities and elements over toward the right-hand side (non-metals) have higher electronegativities. There are several ways to calculate electronegativities but in general it is not very useful to memorize specific numbers. It is helpful, however, to understand the trends and to be able to predict bond polarities. Because fluorine is the most electronegative element it can be expected to make the most polarized bonds with hydrogen.[10] So let us take this logic a bit further. If HF has the most polar bonds then HF molecules should stick together with the strongest attractions and HF should have the highest boiling point. But oh no! Water’s boiling point is significantly higher (\(100 { }^{\circ}\mathrm{C}\) compared to \(19 { }^{\circ}\mathrm{C}\) for \(\mathrm{HF}\)). What is going on? Oxygen is not as electronegative as fluorine and so the \(\mathrm{O-H}\) bond is not as polar as the \(\mathrm{H–F}\) bond. Why then is the boiling point of \(\mathrm{H}_{2}\mathrm{O}\) \(81 { }^{\circ}\mathrm{C}\) higher than \(\mathrm{HF}\)? To answer this question we need to consider another factor that affects the polarity of a molecule – and that is molecular shape.
Questions to Answer
- Why do you think that the trends in effective nuclear charge, ionization energy, and electronegativity are correlated?
- What does correlated mean?
- Can you draw a picture of (say) four \(\mathrm{H-F}\) molecules sticking together?
- Is there any arrangement that they might take up or would they stick together in a totally random way?
Questions to Ponder
- Why would you not expect polymeric oxygen, that is molecules similar to hydrocarbon chains (or perhaps you would)?


