10.4: Solutions to Selected Problems
- Page ID
- 189984
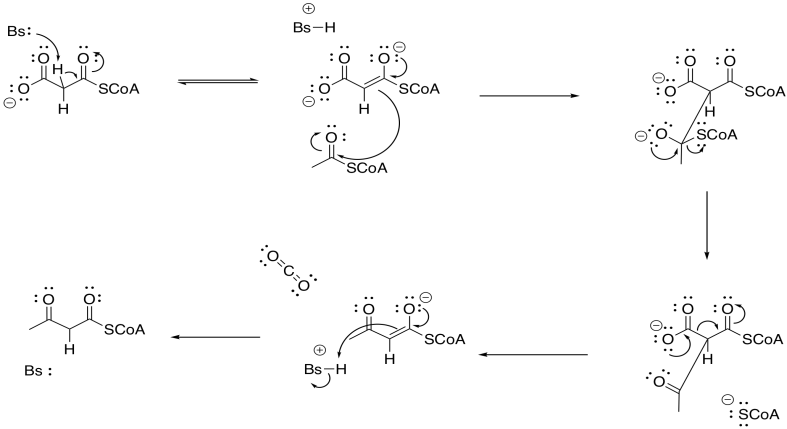
Exercise 10.2.1:
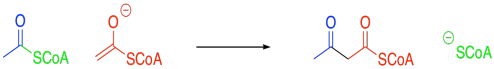
Exercise 10.2.2
The product becomes conjugated. In general, the more conjugation there is in the product of an aldol addition, the more likely is a subsequent condensation (elimination or dehydration). However, other conditions can lead to the loss of water.
Exercise 10.2.3:
Entropy. The dehydration or elimination takes one molecule (the beta-hydroxy thioester) and converts it into two molecules (the water and the alpha,beta-unsaturated thioester. That change represents an increase in internal entropy. Because the entropy term in free energy is weighted by temperature (ΔG = ΔH - TΔS), it predominates as the temperature rises.
Exercise 10.3.1:
In the case of the malonyl enolate, there is additional delocalisation as demonstrated by resonance. This anion has extra stability.
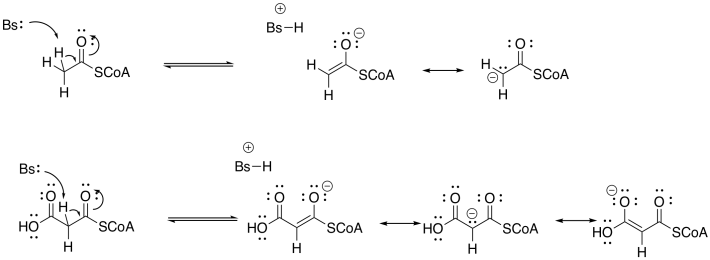
Exercise 10.3.2: