7.12: Ion Exchange Chromatography
- Page ID
- 195355
In ion exchange chromatography, we can separate ions from each other, largely based on their relative charges. If one ion is more highly charged than another, it will have more affinity for the stationary phase in an ion exchange column. It will spend more time on the stationary phase and move more slowly. A compound with a lower charge will elute first.
The stationary phase would have charges opposite those of the ions that we wished to separate. For example, maybe the column is packed with a phase that contains many anions. Maybe we have some sulfate ions attached to the packing in the column, each with a negative charge.

Suppose we have some cationic coordination complexes that we wish to separate from each other. We can dissolve them in water and introduce them to the column. Some of them have a charge of +1 and some of them have a charge of +2. If we introduced them to a stationary phase that contained a lot of anions, they both would stick to the stationary phase. The cations with the +2 charge would stick more strongly, however, and take longer to elute from the column. They would spend more time sitting still in the stationary phase and less time moving along in the mobile phase.
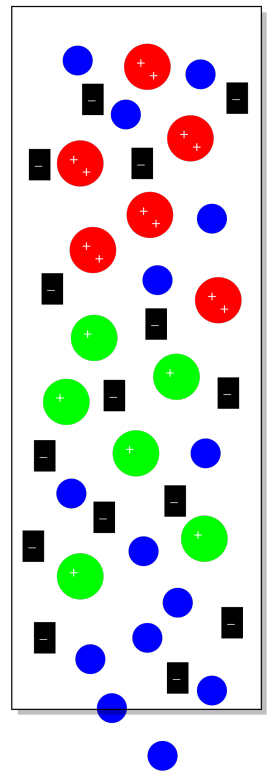
Of course, there are some real problems with the picture so far. First of all, we can never have just one ion without a counterion. So those sulfate ions in the original stationary phase would have sodium ions, for example, and the sodium ions would balance out the charge.
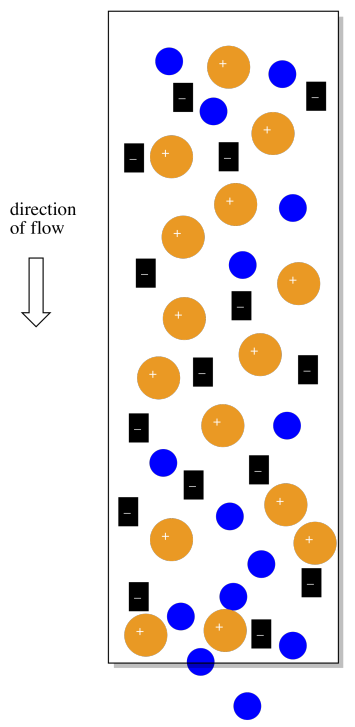
The sodium ions will be very helpful in another way. If there are no counterions, why would the cationic complexes ever come off the column? They would just stick there forever. However, if the mobile phase is a salt solution, containing more sodium ions, then eventually the sodium ions will displace the coordination complexes, which will move along in the mobile phase for awhile.
Practically speaking, the eluted fractions will not contain pure coordination complexes. They will contain the mobile phase they came with when they eluted off the column, and that mobile phase contains some sodium salts. However, each coordination complex will be separate from the other one.


