6.3: GC-MS and LC-MS
- Page ID
- 189784
There are other aspects of this experiment that have been left out of this description. For example, the experiment is done in a vacuum with a low concentration of sample. Among other reasons, these steps are taken to lower the population of molecules in the instrument; that decreases the chance that ions and molecules will bump into each other and undergo reactions with each other, which might lead to the formation of larger ions with all sorts of different masses.
However, there is one important instrumental consideration to discuss. In mass spectrometry, the sample can be purified immediately after it is introduced into the instrument to make sure that only one kind of molecule is being analysed at a time. This purification is accomplished through chromatography, and so mass spectrometry when done in this way is often referred to as "GC-MS" or "LC-MS", depending on the type of chromatography used.
GC-MS employs gas chromatography, sending components through a chromatography column that is heated in an oven so that compounds can be separated out largely based on their boiling points. LC-MS uses liquid chromatography, in which components are most often separated because of polarity differences, although other criteria can be utilized as well, based on the type of packing material that is used in the chromatography column.
The other advantage of this approach is that several different components of one mixture can be analysed one by one.
The GC part of a GC-MS, termed the chromatograph, generates a plot called a chromatogram.
- The chromatogram is a graph showing amount of molecules on the y axis and time on the x-axis.
- Retention time is the amount of time it takes a compound to move through the GC.
- Different compounds have different retention times based on their physical properties.
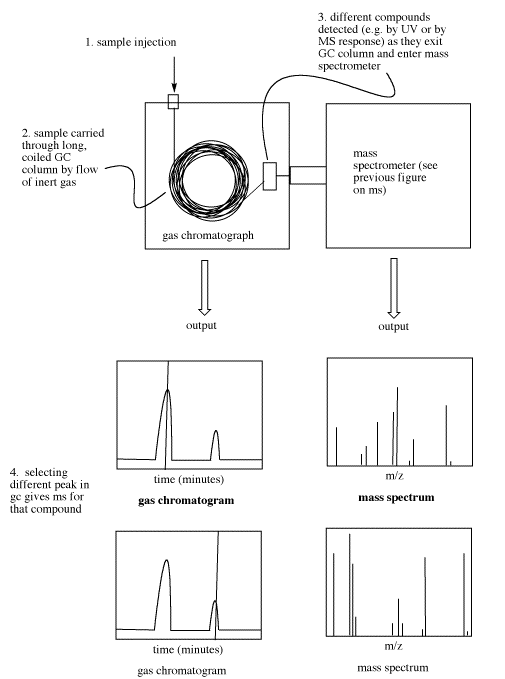
Retention time refers to how long it takes different compounds to emerge from the chromatography column. Peaks are seen on this plot whenever a compound enters the mass spectrometer. The mass spectrometer runs continuously and records whatever masses it detects at any time, so if a student selects a peak in the chromatogram on the computer screen, the corresponding mass spectrum can be shown.
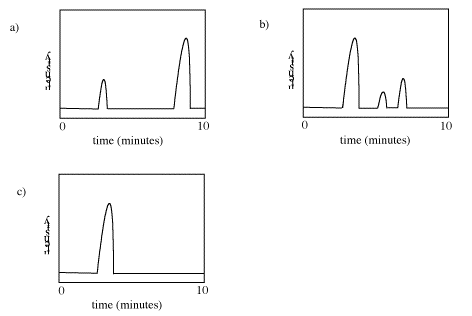
How many compounds appear to be present in each of the following gas chromatograms?