5.7: 2D NMR Solutions
- Page ID
- 195117
Exercise 5.1.1:
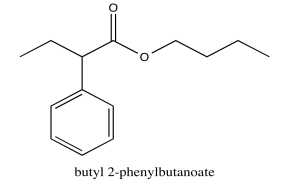
ethyl butanoate
Exercise 5.1.2:
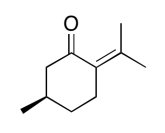
Exercise 5.1.3:
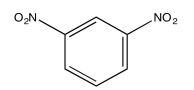
Exercise 5.1.4:
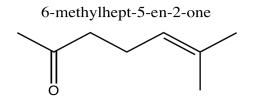
Exercise 5.1.5:
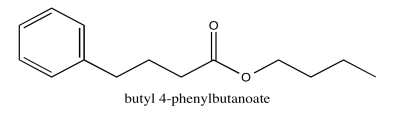
Exercise 5.1.6:
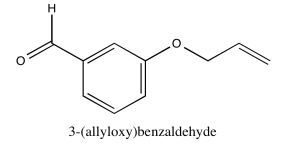
Exercise 5.1.7:
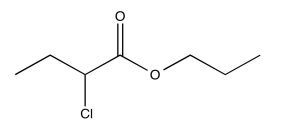
Exercise 5.1.8:
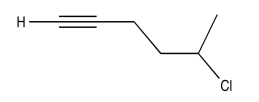
Exercise 5.1.9:
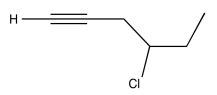
Exercise 5.1.10:
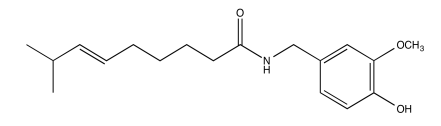
Exercise 5.1.11:
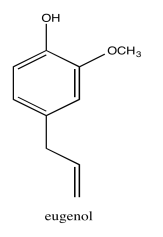
Exercise 5.1.12:
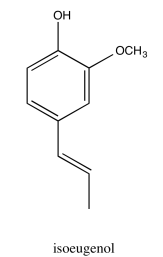
Exercise 5.2.1:
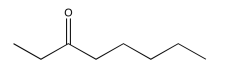
Exericse 5.2.2:
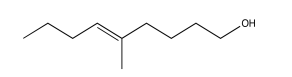
Exercise 5.2.3:
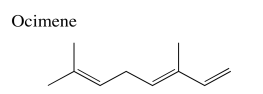
Exercise 5.3.1:
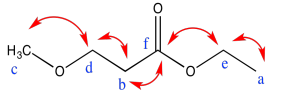
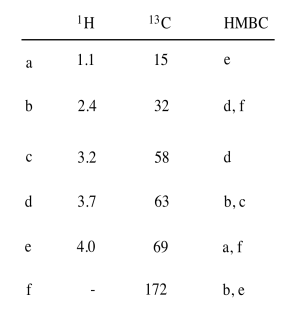
Exercise 5.3.2:
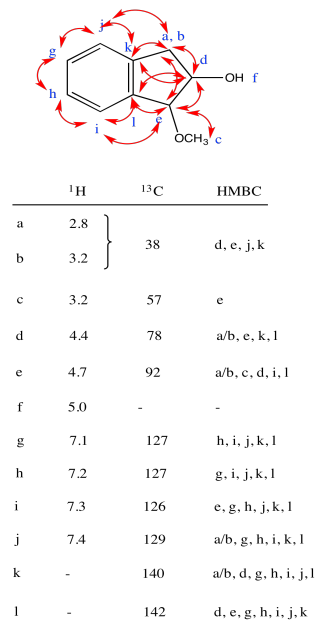
Exercise 5.3.3:
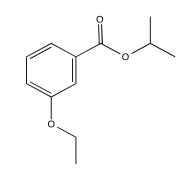
Exercise 5.3.4:
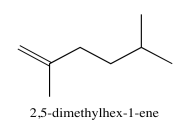
Exercise 5.3.5:
Exercise 5.3.6:
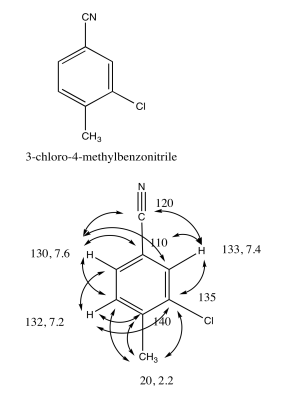
Exercise 5.3.7:
Exercise 5.3.8:
Exercise 5.4.1:
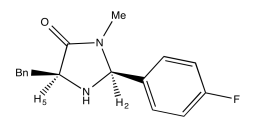
Exercise 5.4.2:
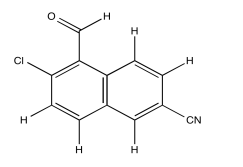
Exercise 5.4.3:
a) Due to resonance, there is substantial pi character to the amide bond which restricts free rotation around that bond.
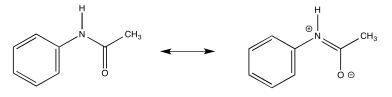
b)
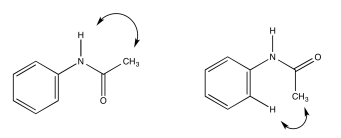
c)
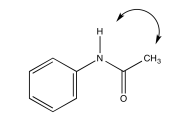
Exercise 5.4.4:
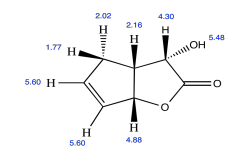
Exercise 5.4.5:
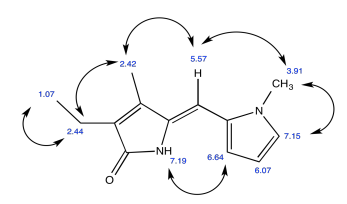
Exercise 5.4.6:
a)
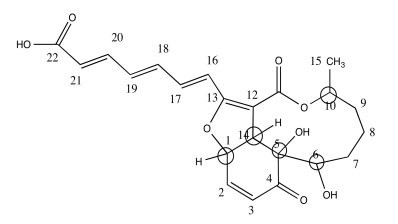
b)
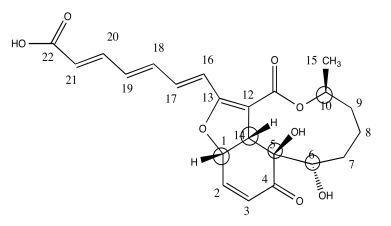
c)
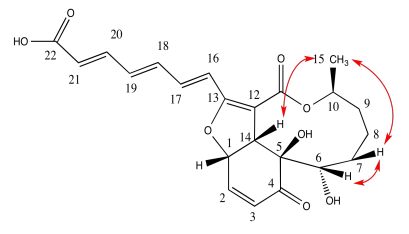
Exercise 5.4.7:
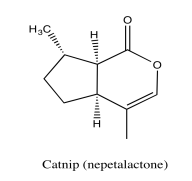
Exercise 5.5.1:
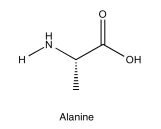
Exercise 5.5.2:
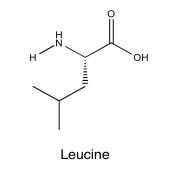
Exercise 5.5.3:
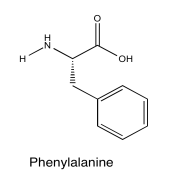
Exercise 5.5.4:
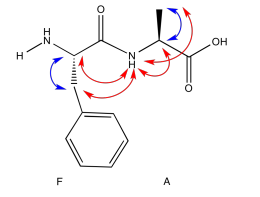
Exercise 5.5.5:
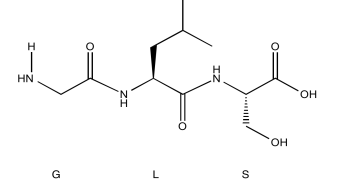
Exercise 5.6.1:
1H NMR
| Chemical shift (ppm) | Integration | Multiplicity | Partial Structure |
| 5.54 | 2H | multiplet | CH=C (x 2) |
| 4.17 | 2H | doublet | O-CH2-CH |
| 2.08 | 2H | quintet | CH-CH2-CH3 |
| 1.47 | 1H | boad singlet | OH |
| 0.95 | 3H | triplet | CH2-CH3 |
* total # H: 10
13C NMR
|
Chemical shift (ppm) |
Type of carbon |
| 134 | sp2 |
| 128 |
sp2 |
| 58 | sp3-O |
| 21 | sp3 |
| 14 | sp3 |
*total # C: 5
COSY
| Assignment | 1H | COSY |
| A | 0.95 | 2.08 |
| B | 2.08 | 0.95, 5.54 |
| C | 4.17 | 5.54 |
| D | 5.54 | 2.08 |
| E | 5.54 | 4.17 |
*HMQC indicates two hydrogens at 5.54 are in two different environments
HMQC
| Assignment | 13C | 1H |
| A | 14 | 0.95 |
| B | 21 | 2.08 |
| C | 58 | 4.17 |
| D | 128 | 5.54 |
| E | 134 | 5.54 |
Formula:
C5H10O (1 O indicated from shift in 13C, 1H NMR)
FW = \(5 \times 12) + (10 \times 1) + (1 \times 16) = 86\)
Compare C5H10 ratio to C5H12 in hydrocarbon
Degrees of unsaturation = \(\frac{(2 \times 5) + 2 - 10}{2} = 1 unit (1 double bond)
The data tables should be consitent with this structure:
pent-2-en-1-ol (could be cis or trans based on this analysis)
Exercise 5.6.2:
1H NMR:
| Chemical shift (ppm) | Integration | Multiplicity | Partial structure |
| 4.7 | 5H | singlet | solvent |
| 3.93 | 1H | triplet | CH2-CH-N |
| 2.40 | 2H | multiplet | CH2-CH2? |
| 2.09 | 2H | multiplet | CH2-CH2? |
| 1.4 | 9H | singlet | C(CH3)3 |
*Total number of H: 19 H
13C NMR:
| Chemical shift (ppm) | Type of carbon |
| 170 | sp2 (C=O) |
| 80 | sp3 (C-O) |
| 52 | sp3 (C-N) |
| 32 | sp3 |
| 28 | sp3 |
| 26 | sp3 |
*Total number of C: 6 apparent, but two more suggested by symmetry (3 methyl groups in 1H NMR) for 8 C; a third extra suggested by MW fit for 9 C
COSY:
| Assignment | 1H | COSY |
| Solvent | 4.7 | -- |
| B | 3.93 | 2.40 |
| D | 2.40 | 2.09 |
| C | 2.09 | 2.40, 2.09 |
| A | 1.4 | -- |
Formula:
C9H18O3N2 (extra O indicated from shift in 13C, 1H NMR; second O suggested by C=O in 13C NMR; additional CO needed to fit MW)
FW = \((9 \times 12) + (18 \times 1) + (3 \times 16) + (2 \times 14) = 202\)
FW = ((9 \times 12) + (18 \times 1) + (3 \times 16) + (2 \times 14) = 202\)
Compare C9H18 to C9H22 for the corresponding hydrocarbon corrected for two nitrogens (therefore two extra hydrogens)
Degrees of unsaturation = \(\frac{(2 \times 9) + 2 + 2 - 10}{2} = 2\) units (2 double bonds)
The data tables should be consistent with this structure:
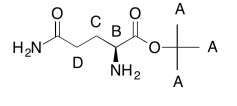
Exercise 5.6.3:
The data should be consistent with this structure:
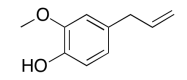
Exercise 5.6.4:
1H NMR:
|
Chemical shift (ppm) |
Integration | Multiplicity | Partial Structure |
| 4.71 | -- | singlet | solvent |
| 4.17 | 1H | doublet? | CO-CH-N |
| 3.75 | 3H | singlet | O-CH3 |
| 2.25 | 1H | multiplet | CH-CH-(CH3)2 |
| 0.92 | 6H | triplet? | 2 x CH3 |
13C NMR:
| Chemical shift (ppm) | Type of carbon |
| 170 | sp2 C=O |
| 60 | sp3 C-N |
| 52 | sp3 C-O |
| 30 | sp3 C |
| 19 | sp3 C |
COSY:
| Assignment | 1H | COSY |
| A | 4.17 | 2.25 |
| B | 3.75 | -- |
| C | 2.25 | 4.17 |
| D | 0.92 | 2.25 |
Formula:
C6H13O2N (1 O indicated from shift in 13C, 1H NMR)
FW = \((6 \times 120 + (13 \times 1) + (2 \times 16) + (1 \times 14) = 131\)
Degrees of unsaturation = \(\frac{(2 \times 6) + 2 + 1 - 13}{2} = 1\) unit (1 double bond)
The data should be consistent with this structure:
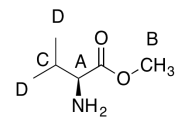
Exercise 5.6.5:
The data should be consistent with this structure:
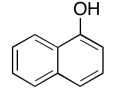
Exercise 5.6.6:
The data should be consistent with this structure:
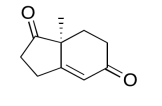
Exercise 5.6.7:
The data should be consistent with this structure:
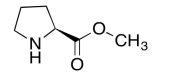
Exercise 5.6.8:
The data should be consistent with this structure:
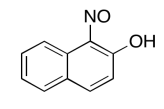
Exercise 5.6.9:
The data should be consistent with this structure:
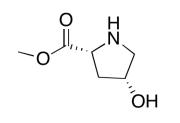
Exercise 5.6.10:
The data should be consistent with this structure:
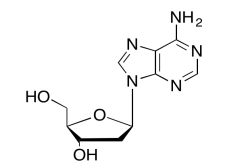
Exercise 5.6.11:
The data should be consistent with this structure:
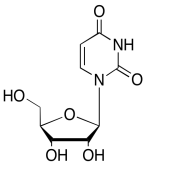
Exercise 5.6.12:
The data should be consistent with this structure:
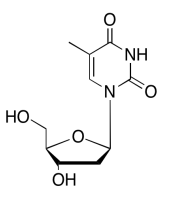
Exercise 5.6.13:
1H NMR:
| Chemical shift (ppm) | Integration | Multiplicity | Partial structure |
| 5.7 | 1H | singlet | C=CH-CO |
| 2.77 | 1H | multiplet | CH2-CH-CO |
| 2.62 | 1H | multiplet | C-CH-C |
| 2.39 | 1H | multiplet | C-CH-C |
| 2.05 | 1H | doublet? | C-CH-CH? |
| 1.96 | 3H | singlet | C-CH3 |
| 1.48 | 3H | singlet | C-CH3 |
| 0.98 | 3H | singlet | C-CH3 |
13C NMR:
| Chemical shft (ppm) | Type of carbon |
| 204 | sp2 C=O |
| 170 | sp2 |
| 121 | sp2 |
| 59 | sp3 |
| 55 | sp3 |
| 50 | sp3 |
| 41 | sp3 |
| 28 | sp3 |
| 24 | sp3 |
| 22 | sp3 |
COSY:
| Assignment | 1H | COSY |
| 1 | 2.39 | 2.77, 2.62? |
| 3 | 5.7 | 2.05? |
| 5 | 2.62 | 2.62? |
| 7a | 2.77 | 2.05 |
| 7b | 2.05 | 2.77 |
| 8 | 1.48 | -- |
| 9 | 0.98 | -- |
| 10 | 1.96 | -- |
Formula:
C10H14O (1 O indicated from shift in 13C, 1H NMR)
FW = \((10 \times 12) + (14 \times 1) + (1 \times 16) = 150 \)
Degrees of unsaturation = \(\frac{(2 \times 10) + 2 - 14}{2} = 4 units (e.g. 2 rings, 2 double bonds)
The data should be consistent with this structure:
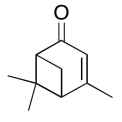
Exercise 5.6.14:
The data should be consistent with this structure:
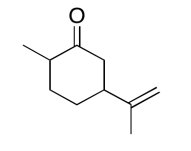
Exercise 5.6.15:
The data should be consistent with this structure: