4.14: NMR in Lab- Monitoring Reaction Progress
- Page ID
- 194887
If you are running a reaction, chances are you know what you started with. You may also have a pretty good idea of what you will be making. The big question is whether the reaction worked (or, maybe, nothing happened at all). If it worked, did you get the product you thought you would, or did you get something unexpected?
In these cases:
- You should draw attention to pieces of data that most strongly support your expected structure. This approach will demonstrate evaluative understanding of the data; that means you can look at data and decide what parts are more crucial than others.
- You should also draw attention to negative results: that is, peaks that might be there if this spectrum matched another, possible structure, but that are in fact missing.
One of the most complicated problems to deal with is the analysis of a mixture. This situation is not uncommon when students run reactions in lab and analyse the data.
- Sometimes the spectra show a little starting material mixed in with the product.
- Sometimes spectra show only a little product and a lot of starting material.
- In these cases, you should probably make two completely separate sets of data tables for your analysis, one for each compound, or else one for the main compound and one for impurities.
Let's look at an example of a reaction. A student is running a type of reaction called an oxidation. She is starting with 3-methoxybenzyl alcohol and trying to convert it to 3-methoxybenzaldehyde. She follows the directions carefully, but how will she know whether she is successful?
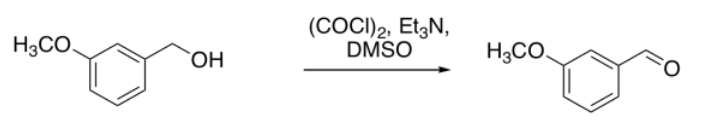
She looks at the spectrum, and sees a group of aromatic hydrogens, and a large singlet near 4 ppm, which could be the hydrogens on the methoxy group. Those are reassuring, but they don't interest her that much, because they would appear in her spectrum whether the reaction had proceeded or not. Those peaks would all be pretty much the same in her starting material.
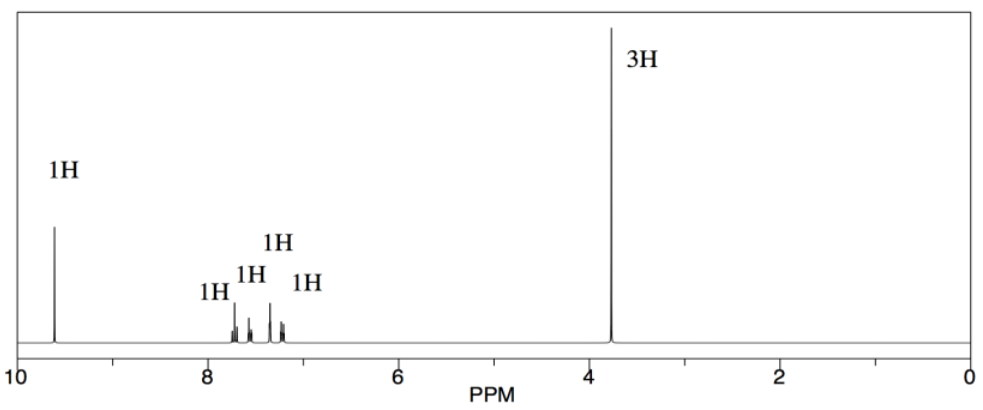
Instead, she notices the peak near 10 ppm. That's significant. Her product would have an aldehyde proton, and that would show up around 10 ppm. Maybe the reaction worked.
She also takes a look around 5 ppm. There is nothing there, and she thinks that's interesting, too. In her starting alcohol, she had protons on a carbon that was in between an arene and an oxygen, Ar-CH2-OH. She knows that shift effects are additive in NMR: if a CH2 in general shows up around 1 ppm, but next to an oxygen it gets shifted to 4 ppm and next to an aromatic it gets shifted to 2 ppm, then if it is next to both it will shift to 5 ppm. There is nothing there. She no longer has an alcohol.
She makes an argument in her report that the reaction proceeded to form an aldehyde. She does not have the hubris to call the reaction "successful" or say it went "as expected". She just points out the presence of an aldehyde proton and the absence of the protons next to the alcohol. She gets an A on the report. Three years later, she is in medical school at Harvard.
Sometimes, things are more subtle. Let's look at another reaction. A student is performing an esterification reaction. He treats 1-octanol with benzoic acid and a drop of sulfuric acid. It's a Fischer esterification. He does not realize Fischer esterifications are equilibrium quagmires that never really make it one direction or another.
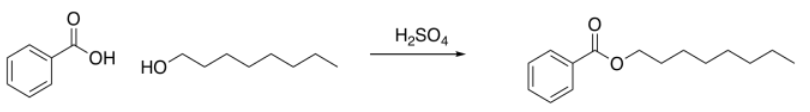
But when he sees the results, he suspects something is amiss. All the peaks are there -- the aromatics, the -CH2-O, the long aliphatic chain -- but there is something about that methylene, that -CH2-O, that bothers him. It shows up right around 3.5 ppm, exactly where it would in the starting alcohol. He thinks it should be just a little to the left in the ester, maybe around 4 ppm, because of the influence of the electron-withdrawing carbonyl. He thinks maybe the reaction did not proceed. It occurs to him to look for evidence of the OH protons, in both the alcohol and the benzoic acid, but he knows looking for that in the 1H spectrum is usually a fool's errand, so he consults the IR evidence instead.
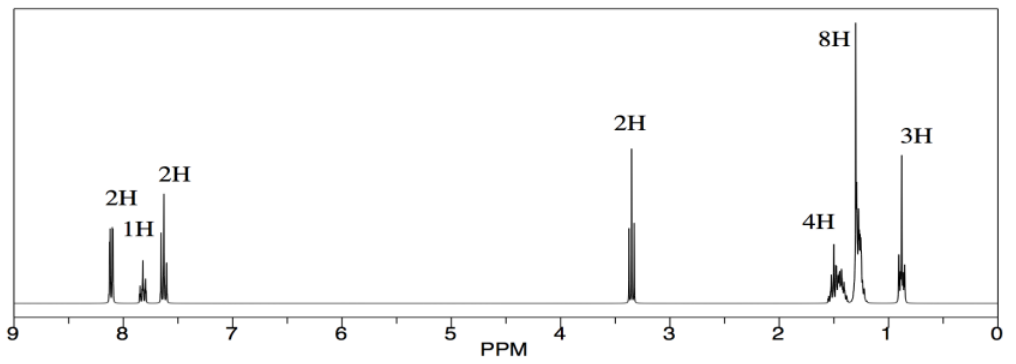
He makes an argument in the report that the reaction did not proceed, but yielded unreacted starting materials. He does not wring his hands about human error, but points out that esters can be both formed and cleaved in the presence of acid, and suggests an acid chloride route might be preferable. He still manages to get an A on the report.
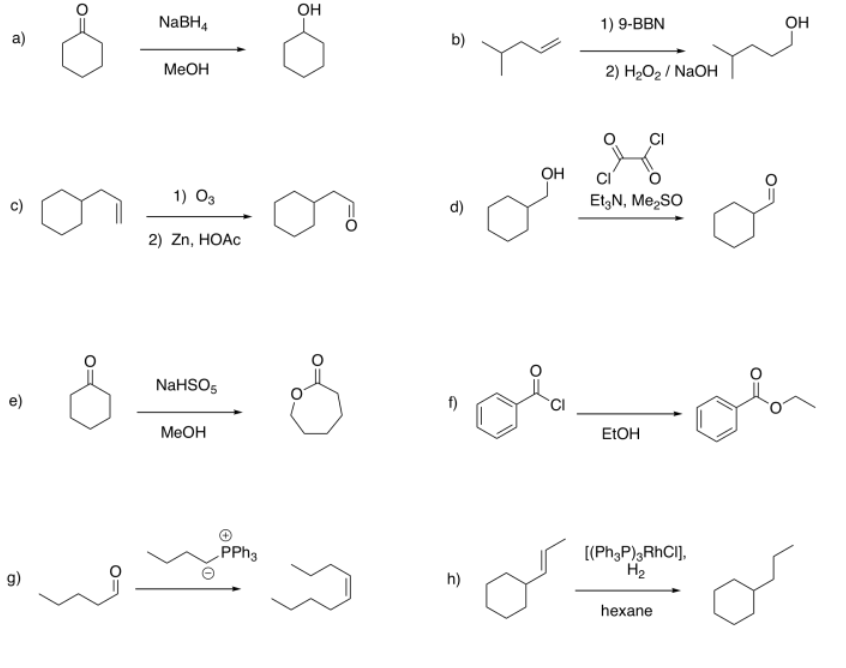
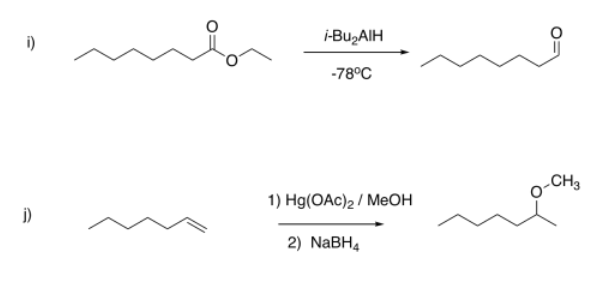
Suggest diagnostic NMR peaks that could be used to determine whether each of the following reactions has proceeded.
- Answer
-
a) appearance of CH-O near 3.5 ppm (multiplet, 1H)
b) appearance of CH2-O near 3.5 ppm (triplet, 2H); disappearance of =CH near 5-6 ppm (mutiplets, total 3H)
c) appearance of HC=O near 10 ppm (triplet, 1H); disappearance of =CH near 5-6 ppm (mutiplets, total 3H)
d) appearance of HC=O near 10 ppm (triplet, 1H); disappearance of CH2-O near 3.5 ppm (doublet, 2H)
e) appearance of CH2-O near 3.5 ppm (triplet, 2H)
f) appearance of CH2-O near 4 ppm (triplet, 2H)
g) appearance of =CH near 5-6 ppm (mutiplets, total 2H); disappearance of HC=O near 10 ppm (triplet, 1H);
h) disappearance of =CH near 5-6 ppm (mutiplets, total 2H); appearance of triplet:sextet:triplet pattern between 1-2 ppm
i) appearance of HC=O near 10 ppm (triplet, 1H); disappearance of CH2-O near 3.5 ppm (doublet, 2H)
j) appearance of CH-O near 3 ppm (singlet, 3H) and 3.5 ppm (sextet, 1H); disappearance of =CH near 5-6 ppm (mutiplets, total 2H)


