4.10: Multiplicity
- Page ID
- 194880
Another type of additional data available from 1H NMR spectroscopy is called multiplicity or coupling. Coupling is useful because it reveals how many hydrogens are on the next carbon in the structure. That information helps to put an entire structure together piece by piece.
In ethanol, CH3CH2OH, the methyl group is attached to a methylene group. The 1H spectrum of ethanol shows this relationship through the shape of the peaks. The peak near 3.5 ppm is the methylene group with an integral of 2H.
- This peak is split into four smaller peaks, evenly spaced, with taller peaks in the middle and shorter on the outside.
- This pattern is called a multiplet, and specifically a quartet. A quartet means that these hydrogens have three neighbouring hydrogens on adjacent carbons.
Source: Modified from SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 15 August 2008)
The integral of 2H means that this group is a methylene, so it has two hydrogens. The carbon bearing these two hydrogens can have two other bonds.
There could be two hydrogens on one neighbouring carbon and one on another. Otherwise, all three hydrogens could be on one neighbouring carbon.
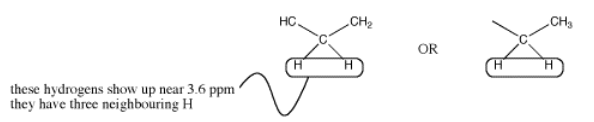
However, the shift of 3.5 ppm means that this carbon is attached to an oxygen. Mutliplicity usually only works with hydrogens on neighbouring carbons. If there is an oxygen on one side of the methylene, all three neighbouring hydrogens must be on a carbon on the other side.
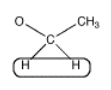
Alternatively, look at the spectrum the other way around. The peak at 1 ppm is the methyl group with an integral of 3H.
- The peak is split into three smaller ones, evenly spaced, with a taller one in the middle and shorter ones on the outside.
- This pattern is called a triplet. A triplet means that these hydrogens have two neighbouring hydrogens on adjacent carbons.
The neighbouring H could be on two different neighbouring carbons or both on the same one.
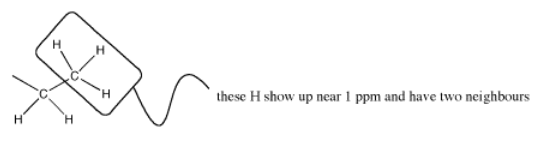
But this group is a methyl; the carbon already has three bonds, so it can have only one neighbouring carbon. It is next to a methylene group.
The number of lines in a peak is always one more than the number of hydrogens on the neighboring carbon. The triplet for the methyl peak means that there are two neighbors on the next carbon (3 - 1 = 2H); the quartet for the methylene peak indicates that there are three hydrogens on the next carbon (4 - 1 = 3H). Table NMR 1 summarizes coupling patterns that arise when protons have different numbers of neighbors.
|
# of lines |
ratio of lines |
term for peak |
# of neighbors |
|
1 |
- |
singlet |
0 |
|
2 |
1:1 |
doublet |
1 |
|
3 |
1:2:1 |
triplet |
2 |
|
4 |
1:3:3:1 |
quartet |
3 |
|
5 |
1:4:6:4:1 |
quintet |
4 |
|
6 |
1:5:10:10:5:1 |
sextet |
5 |
|
7 |
1:6:15:20:15:6:1 |
septet |
6 |
|
8 |
1:7:21:35:35:21:7:1 |
octet |
7 |
|
9 |
1:8:28:56:70:56:28:8:1 |
nonet |
8 |
The third peak in the ethanol spectrum is usually a "broad singlet". This is the peak due to the OH. You would expect it to be a triplet because it is next to a methylene. Under very specific circumstances, it does appear that way. However, coupling is almost always lost on hydrogens bound to heteroatoms (OH and NH). The lack of communication between an OH or NH and its neighbors is related to rapid proton transfer, in which that proton can trade places with another OH or NH in solution. This exchange happens quite easily if there are even tiny traces of water in the sample.
In summary, multiplicity or coupling is what we call the appearance of a group of symmetric peaks representing one hydrogen in NMR spectroscopy.
- A proton can absorb at different frequencies because of the influence of neighbouring hydrogens.
- Protons on one carbon atom are affected by different protons on the next carbon atom, provided those two carbons are directly attached to each other.
- Stated another way, these neighboring hydrogens must be three bonds away (and so this phenomenon is sometimes called "three-bond coupling").
- When a proton is coupled, the number of neighbouring hydrogens is one less than the number of peaks in the multiplet.
There are limitations on coupling:
- coupling does not occur between hydrogens of the same type ("equivalent hydrogens"). In the proton spectrum of ethane, CH3-CH3, you would only observe one singlet.
- coupling often does not occur across heteroatoms such as oxygen and nitrogen. The OH peak in ethanol may be a singlet instead of a triplet, although there are two hydrogens on the neighboring carbon. The methylene peak in ethanol may be a quartet instead of a quintet, even though there are actually four neighboring hydrogens: three on the attached methyl and one on the attached hydroxyl
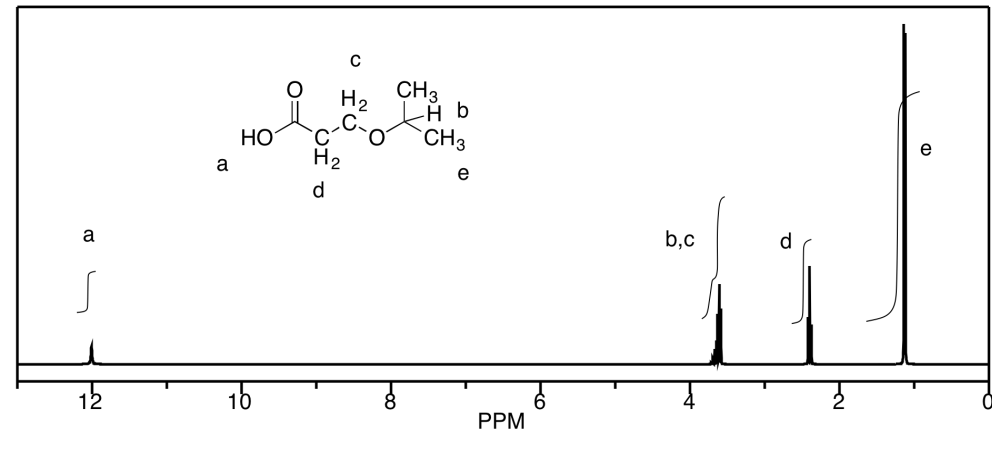
Predict the shift, integration, and multiplicity for the bold hydrogen in each case.
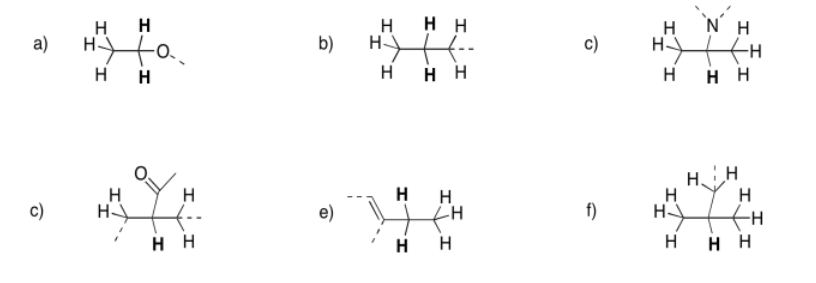
- Answer
-
(There will be some variation in the shift depending on the rest of the structure; this is just an estimate.)
a) 3.5 ppm, quartet, 2H b) 1.5 ppm, sextet, 2H c) 2.6 ppm, septet, 1H
d) 2.3 ppm, quintet, 1H e) 2.2 ppm, quartet, 2H f) 1.7 ppm, nonet, 1H
Predict the shift, integration, and multiplicity for the bold hydrogen in each case.
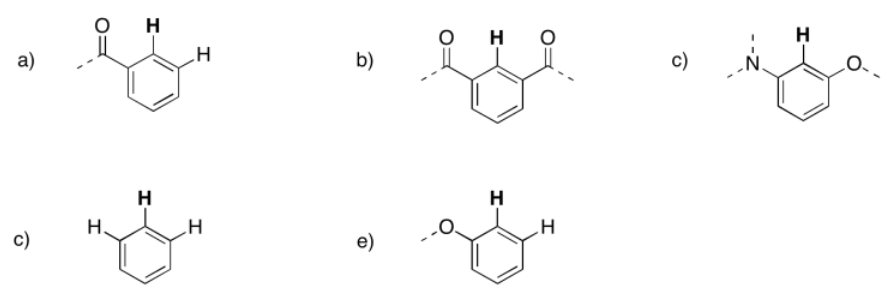
- Answer
-
(There will be some variation in the shift depending on the rest of the structure; this is just an estimate.)
a) 7.8 ppm, doublet, 1H b) 8.4 ppm, singlet, 1H c) 6.7 ppm, singlet, 1H
d) 7.2 ppm, troplet, 1H e) 6.9 ppm, doublet, 1H
The following patterns indicate particular substitution patterns in disubstituted benzenes: 1,2-C6H4XY; 1,3-C6H4XY; or 1,4-C6H4XY. Match each pattern to the correct structure.
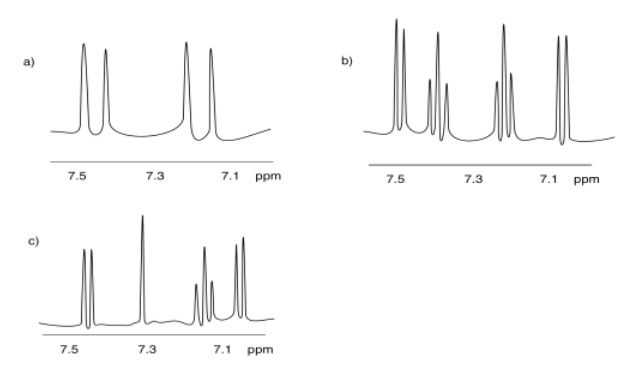
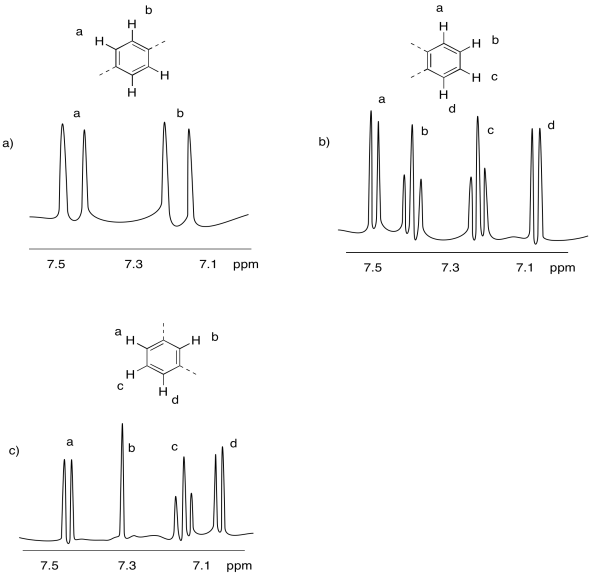
- Answer
-
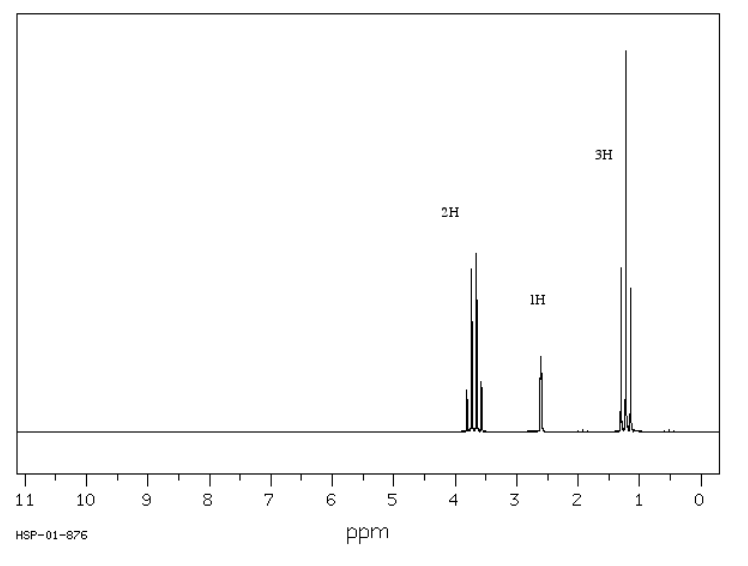
The spectrum of isobutanol is shown below. Assign each peak to a different proton in the structure.
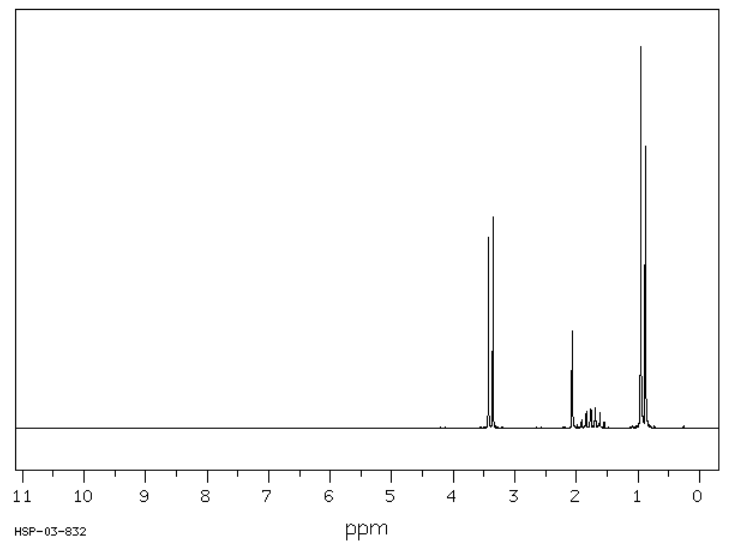
Source: Modified from SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 15 August 2008)
- Answer
-
3.4 ppm, doublet: CH-CH2-O
2.1 ppm, singlet: OH
1.7 ppm, nonet: (CH3)2CHCH2
0.9 ppm, doublet: CH(CH3)2
Sketch predicted 1H NMR spectra, complete with coupling and integration, for the following structures:
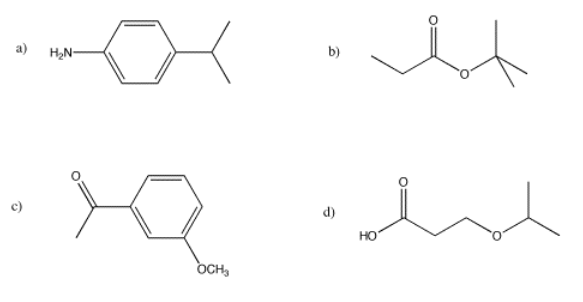
- Answer
-
a) This is a simulated spectrum. The peaks at f, e, and c are singlets. The peak at d is a doublet. The peaks at a & b are unfortunately coincident, so their multiplicities are obscured, but they would be doublets & triplets, respectively.
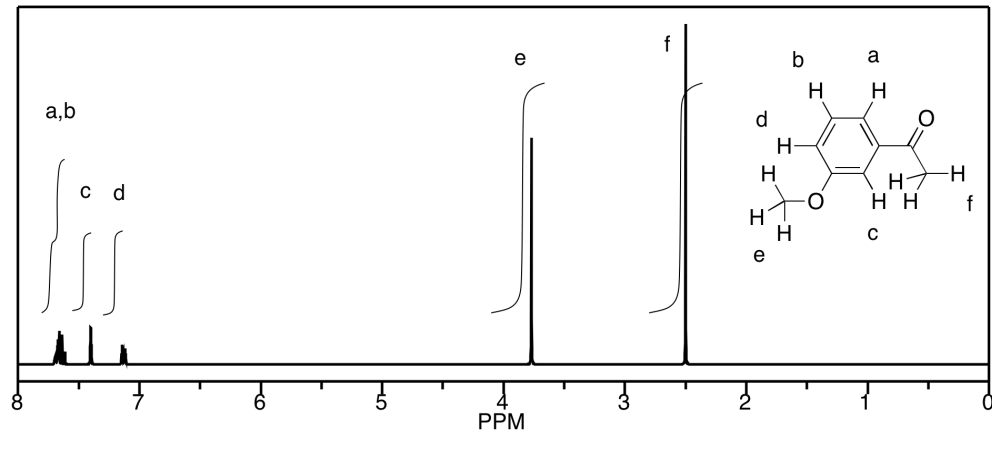
b)
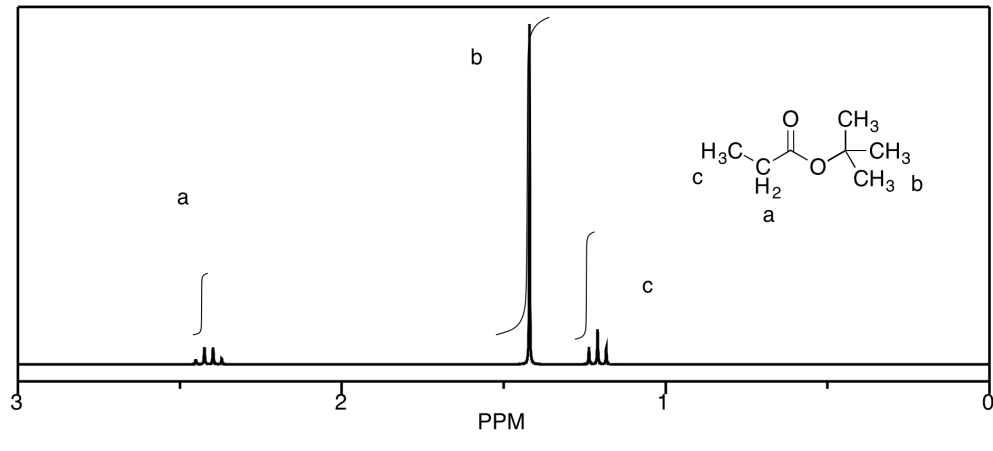
c)
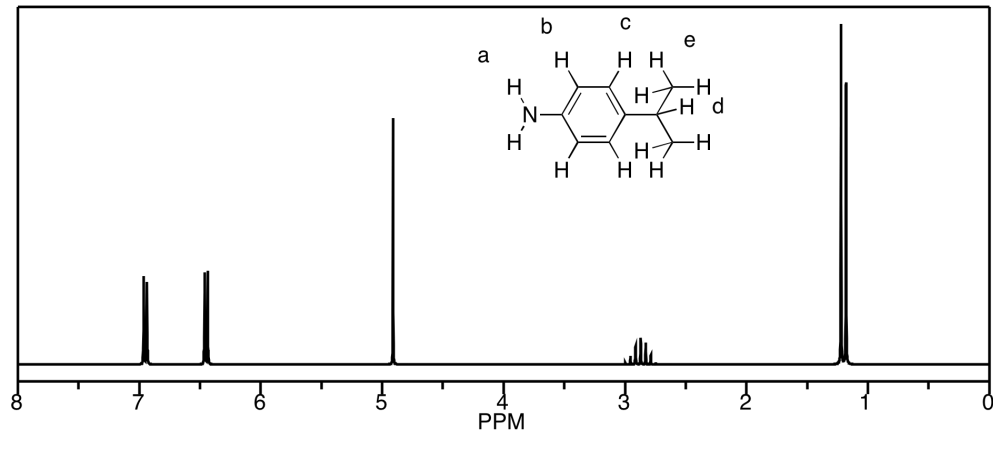
d) The peaks at b and c unfortunately coincide, but they would be a septet and a triplet, respectively. Otherwise, a is a singlet, d is a triplet, and e is a doublet.