3.7: Bonds to Common Heteroatoms- Nitrogen
- Page ID
- 194820
The IR spectra of nitrogen-containing compounds can be messier than the ones you have seen so far. N-H bends and C-N stretches tend to be broader and weaker than peaks involving oxygen atoms. However, some peaks in nitrogen compounds are useful. The problems in this section will guide you through some of these features.
Amines, such as butylamine, have a very diagnostic N-H peak, although the peak is sometimes weak.
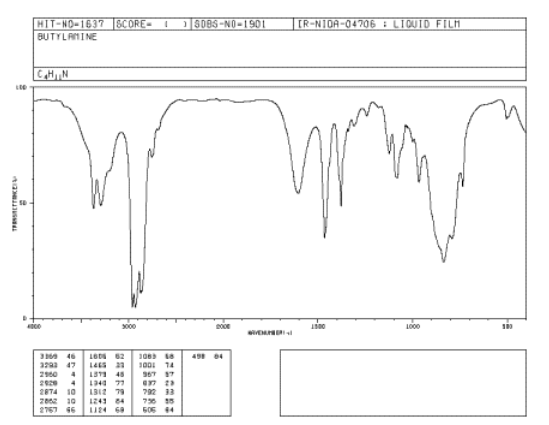
Figure \(\PageIndex{1}\): IR spectrum of butylamine. Source: SDBSWeb: http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)
Butylamine is a primary amine, meaning the nitrogen is attached to one carbon group and two hydrogens. An N-H bond is almost as strong as an O-H bond. Can you identify the N-H peak in this spectrum?
- Answer
-
There are actually two N-H stretching bands near 3400 and 3300 cm-1. This feature is common in NH2 groups; NH groups display only one N-H stretching band.
Dibutylamine is another example of an amine. It is a secondary amine, meaning the nitrogen is attached to one hydrogen and two other groups.
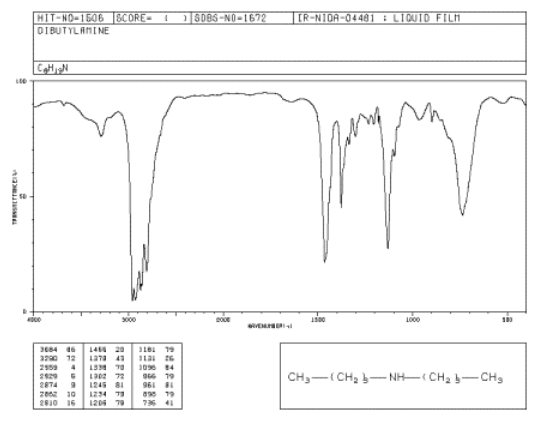
Figure \(\PageIndex{2}\): IR spectrum of dibutylamine. Source: SDBSWeb: http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)
Compare its N-H peak to that of butylamine. What do you notice about the number of N-H peaks in each spectrum?
- Answer
-
There is just one N-H stretching band near 3300 cm-1.
What peaks would you expect to see in the IR spectrum of triisobutylamine?
- Answer
-
This tertiary amine has no N-H bonds. No N-H stretching frequency would appear in the IR spectrum.
Benzonitrile has no N-H bonds but it does have a carbon-nitrogen triple bond that shows up in the IR spectrum. Identify the corresponding peak on the spectrum, plus two other peaks.
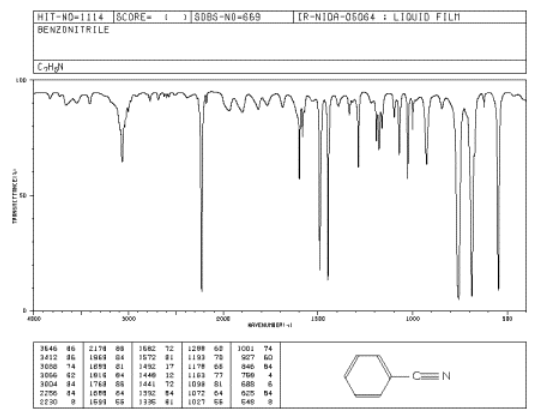
Figure \(\PageIndex{1}\): IR spectrum of benzonitrile. Source: SDBSWeb: http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)
- Answer
-
Figure \(\PageIndex{1}\): IR spectrum of benzonitrile. Source: SDBSWeb: http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)


